Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Proton pump inhibitor; treatment of peptic ulcer disease.
DEFINITION
Esomeprazole Granules contain Esomeprazole Magnesium Trihydrate in a suitable basis. The granules are covered with a gastro-resistant coating.
The granules comply with the requirements stated under Granules and with the following requirements.
Content of esomeprazole, C17H19N3O3S
95.0 to 105.0% of the stated amount.
IDENTIFICATION
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Record the UV spectrum of the principal peak in the chromatograms obtained with solutions (1) and (2) with a diode array detector in the range of 210 to 400 nm.
Solution A 11 volumes of 0.25M trisodium orthophosphate, 22 volumes of 0.5M disodium hydrogen orthophosphate and 67 volumes of water. Adjust to pH 11.0 with orthophosphoric acid or 10M sodium hydroxide.
(1) Shake a quantity of the granules containing the equivalent of 50 mg of esomeprazole in 100 mL of water and filter (a 0.2-mm mesh sieve is suitable). Rinse the granules on the sieve with 0.1mM hydrochloric acid. Disperse the granules in 100 mL of solution A, add 100 mL of water and dilute with ethanol to produce 250 mL and mix. To 1 volume, add 1 volume of water, dilute to 20 volumes with solution A and filter (a 0.45-μm PVDF filter is suitable).
(2) 0.001% w/v of omeprazole BPCRS in solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (10 cm × 4.0 mm) packed with α1-acid-glycoprotein silica gel for chiral separation (5 μm) (Chiralpak AGP is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 0.6 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 302 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
13 volumes of acetonitrile and 87 volumes of a solution containing 0.0025M disodium hydrogen orthophosphate and 0.005 M sodium dihydrogen orthophosphate.
When the chromatograms are recorded under the prescribed conditions, the relative retention with reference to esomeprazole (retention time about 5 minutes) is: (R)-omeprazole (impurity F), about 0.7.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution between the peaks due to (R)- omeprazole and esomeprazole is at least 3.0.
CONFIRMATION
The UV spectrum of the principal peak in the chromatogram obtained with solution (1) is concordant with that of the peak due to esomeprazole in the chromatogram obtained with solution (2);
the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the peak due to esomeprazole in the chromatogram obtained with solution (2).
TESTS
Dissolution
Comply with the dissolution test for tablets and capsules, Appendix XII B1.
Solution A 11 volumes of 0.25M trisodium orthophosphate, 22 volumes of 0.5M disodium hydrogen orthophosphate and 67 volumes of water. Adjust to pH 11.0 with orthophosphoric acid or 10M sodium hydroxide.
Solution B 0.1 volumes of 10M sodium hydroxide and 10 volumes of 0.05M phosphate buffer solution pH 4.5.
Solution C 5.2 volumes of 1M anhydrous sodium dihydrogen orthophosphate and 63.2 volumes of 0.5M anhydrous disodium hydrogen orthophosphate and dilute to 1000 volumes with water. Adjust to pH 7.6 with orthophosphoric acid or 10M sodium hydroxide.
First stage (pH 4.5)
TEST CONDITIONS
(a) Use Apparatus 2, rotating the paddle at 100 revolutions per minute.
(b) Use 700 mL of 0.05M phosphate buffer solution pH 4.5, at a temperature of 37°, as the medium.
PROCEDURE
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) After 60 minutes withdraw 5 mL of the medium and filter (a 0.45-μm nylon filter is suitable). Dilute 1 volume of the filtrate to 5 volumes with solution A and retain the samples for analysis. Proceed immediately to the final stage.
(2) 0.0002% w/v of omeprazole BPCRS in a mixture of 1 volume of solution A and 9 volumes of water.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 2 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Nucleosil C18 is suitable). Use a suitable guard column.
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 0.25 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 302 nm.
(f) Inject 10 μL of each solution.
MOBILE PHASE
25 volumes of solution C, 35 volumes of water and 40 volumes of acetonitrile, adjusted to pH 7.6 with orthophosphoric acid or 10M sodium hydroxide.
When the chromatograms are recorded under the prescribed conditions, the retention time of omeprazole is about 4 minutes.
SYSTEM SUITABILITY
The symmetry factor of the peak due to omeprazole is between 0.8 and 2.0.
DETERMINATION OF CONTENT
Calculate the total content of C17H19N3O3S in the medium using the declared content of C17H19N3O3S in omeprazole BPCRS.
LIMITS
The amount of esomeprazole released is not more than 10% of the stated amount.
Final stage (pH 6.8)
TEST CONDITIONS
(a) Use Apparatus 2, rotating the paddle at 100 revolutions per minute.
(b) Within 1 minute of withdrawing the medium at completion of the first stage, add 200 mL of solution B, at a temperature of 37°, to the vessel.
PROCEDURE
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) After 45 minutes withdraw a sample of the medium and filter (a 0.45-μm nylon filter is suitable). To a volume of the filtrate expected to contain the equivalent of 0.05 mg of esomeprazole, add 1 volume of 0.25M sodium hydroxide and dilute to 25 volumes with solution A.
(2) 0.001% w/v of omeprazole BPCRS in a mixture of 1 volume of solution A and 9 volumes of water.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under the first stage may be used.
SYSTEM SUITABILITY
The symmetry factor of the peak due to omeprazole is between 0.8 and 2.0.
DETERMINATION OF CONTENT
Calculate the total content of C17H19N3O3S, in the medium using the declared content of C17H19N3O3S, in omeprazole BPCRS.
LIMITS
The amount of esomeprazole released is not less than 75% (Q) of the stated amount.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solution A 11 volumes of 0.25M trisodium orthophosphate, 22 volumes of 0.5M disodium hydrogen orthophosphate and 67 volumes of water. Adjust to pH 11.0 with orthophosphoric acid or 10M sodium hydroxide.
(1) Shake a quantity of the granules containing the equivalent of 50 mg of esomeprazole in 100 mL of water and filter (a 0.2 mm mesh sieve is suitable). Rinse the granules on the sieve with 0.1mM hydrochloric acid. Disperse the granules in 100 mL of solution A, add 100 mL of water and dilute with ethanol to produce 250 mL and mix. Dilute 1 volume to 2 volumes with water and filter (a 0.45-μm PVDF filter is suitable).
(2) Dilute 1 volume of solution (1) to 20 volumes with solution A. Dilute 1 volume of this solution to 10 volumes with water.
(3) 0.0001% w/v each of omeprazole BPCRS and omeprazole impurity D EPCRS, prepared by dissolving the reference materials in 1 volume of ethanol, adding 2 volumes of solution A and diluting to 10 volumes with water.
(4) Dilute 1 volume of solution (2) to 5 volumes with water.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (10 cm × 4.6 mm) packed with octylsilyl silica gel for chromatography (5 μm) (Microspher C8 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 302 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Solution D 5.2 volumes of 1.0M sodium dihydrogen orthophosphate monohydrate, 63 volumes of 0.5M disodium hydrogen orthophosphate dihydrate and dilute to 1000 volumes with water. Adjust to pH 7.6 with orthophosphoric acid or 10M sodium hydroxide.
Mobile phase A 100 volumes of acetonitrile, 100 volumes of solution D and dilute to 1000 volumes with water.
Mobile phase B 10 volumes of solution A, 800 volumes of acetonitrile and dilute to 1000 volumes with water.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
|---|---|---|---|
| 0–1 | 100 | 0 | isocratic |
| 1–11 | 100 → 80 | 0 → 20 | linear gradient |
| 11–31 | 80 → 0 | 20 → 100 | linear gradient |
| 31–32 | 0 → 100 | 100 → 0 | linear gradient |
| 32–46 | 100 | 0 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions, the relative retentions with reference to esomeprazole (retention time about 18 minutes) are: impurity 3, about 0.25; impurity 4, about 0.42; impurity 1, about 0.43; impurity A, about 0.5; impurity E, about 0.8; impurity D, about 0.95; impurity 2, about 1.1; impurity C, about 1.2, and impurity G, about 1.4 (2 peaks may be seen).
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution between the peaks due to impurity D and omeprazole is at least 2.5.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity D is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any other secondary peak is not greater than 0.4 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of all secondary peaks is not greater than 4 times the area of the principal peak in the chromatogram obtained with solution (2) (2%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.1%).
ASSAY
Weigh and powder the contents of 20 sachets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solution A 11 volumes of 0.25M trisodium orthophosphate, 22 volumes of 0.5M disodium hydrogen orthophosphate and 67 volumes of water. Adjust to pH 11.0 with orthophosphoric acid or 10M sodium hydroxide.
(1) Shake a quantity of powdered granules containing the equivalent of 50 mg of esomeprazole with 200 mL of solution A, add 50 mL of ethanol and dilute to produce 500 mL with solution A. Dilute 1 volume to 25 volumes with a solution containing 1 volume of ethanol, 4 volumes of water and 20 volumes of solution A and filter (a 1-μm glass fibre filter is suitable).
(2) 0.002% w/v of omeprazole BPCRS in a mixture of 1 volume of ethanol and 4 volumes of solution A. Dilute 1 volume to 5 volumes with water.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Dissolution may be used.
DETERMINATION OF CONTENT
Calculate the content of C17H19N3O3S in the granules using the declared content of C17H19N3O3S in omeprazole BPCRS.
LABELLING
The quantity of active ingredient is stated in terms of the equivalent amount of esomeprazole.
IMPURITIES
The impurities limited by the requirements of this monograph include impurities A, C, D and E listed under Esomeprazole Magnesium Dihydrate and Esomeprazole Magnesium Trihydrate, impurity G listed under Esomeprazole Sodium and:
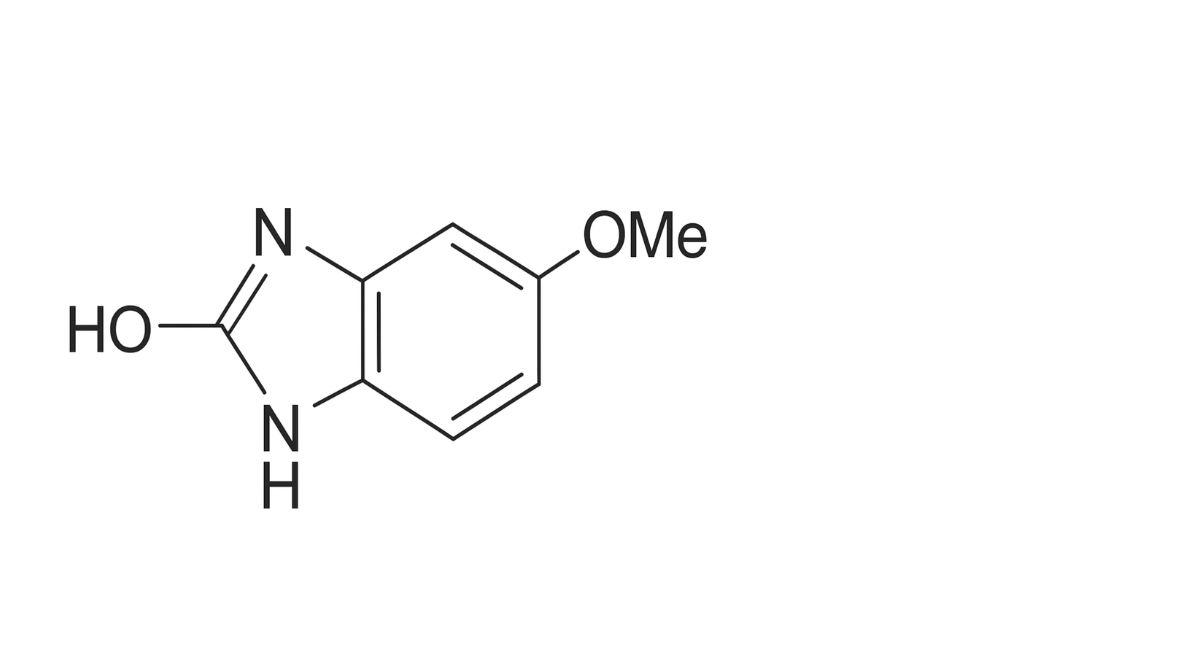
1. 2-hydroxy-5-methoxybenzimidazole (5-methoxy-2-benzimidazolinone),
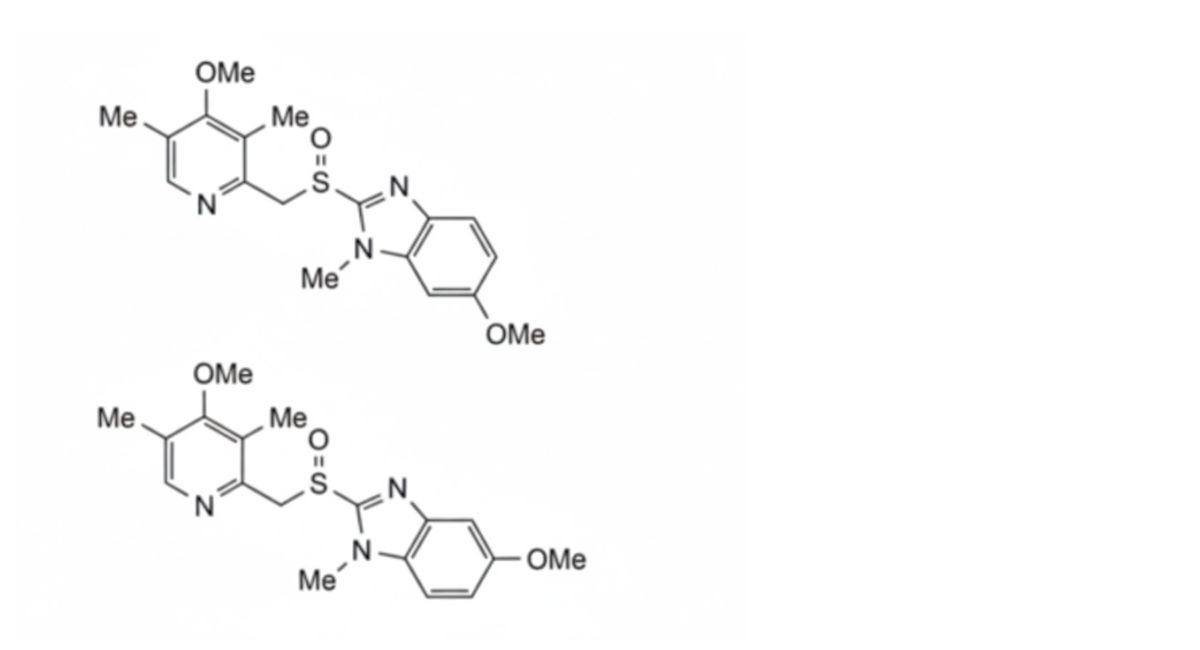
2. 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1-methyl-1H-benzimidazole and 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1-methyl-1H-benzimidazole, regioisomers,
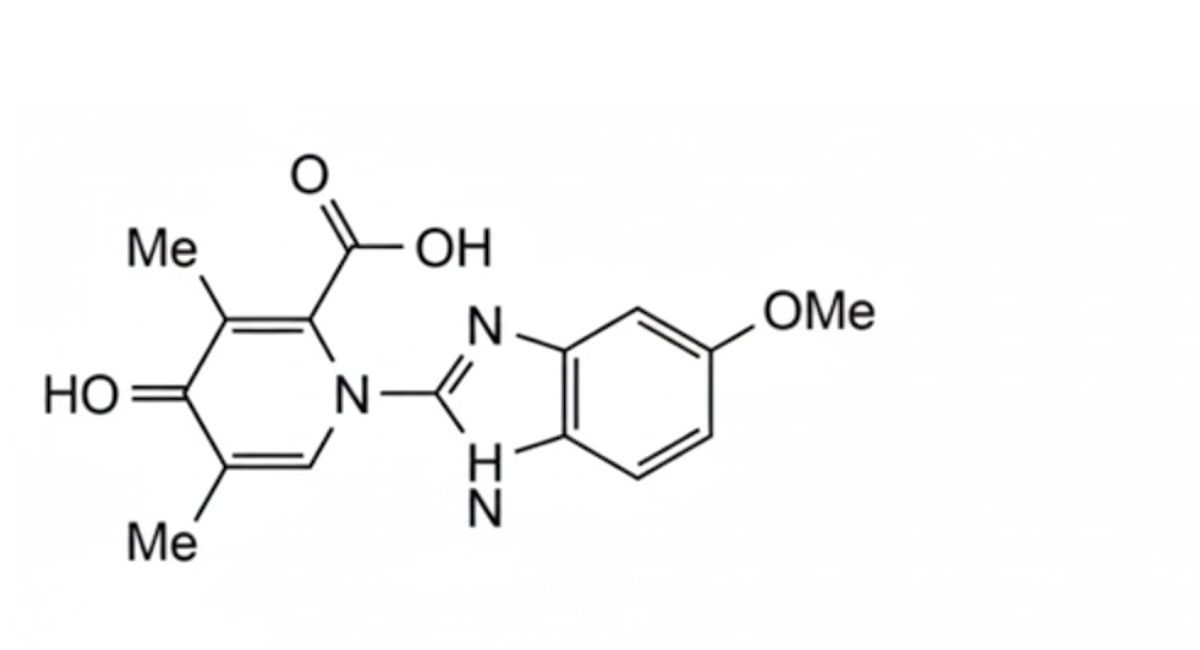
3. 1,4-dihydro-1-(5-methoxy-1H-benzimidazol-2-yl)-3,5-dimethyl-4-oxo-2-pyridinecarboxylic acid,
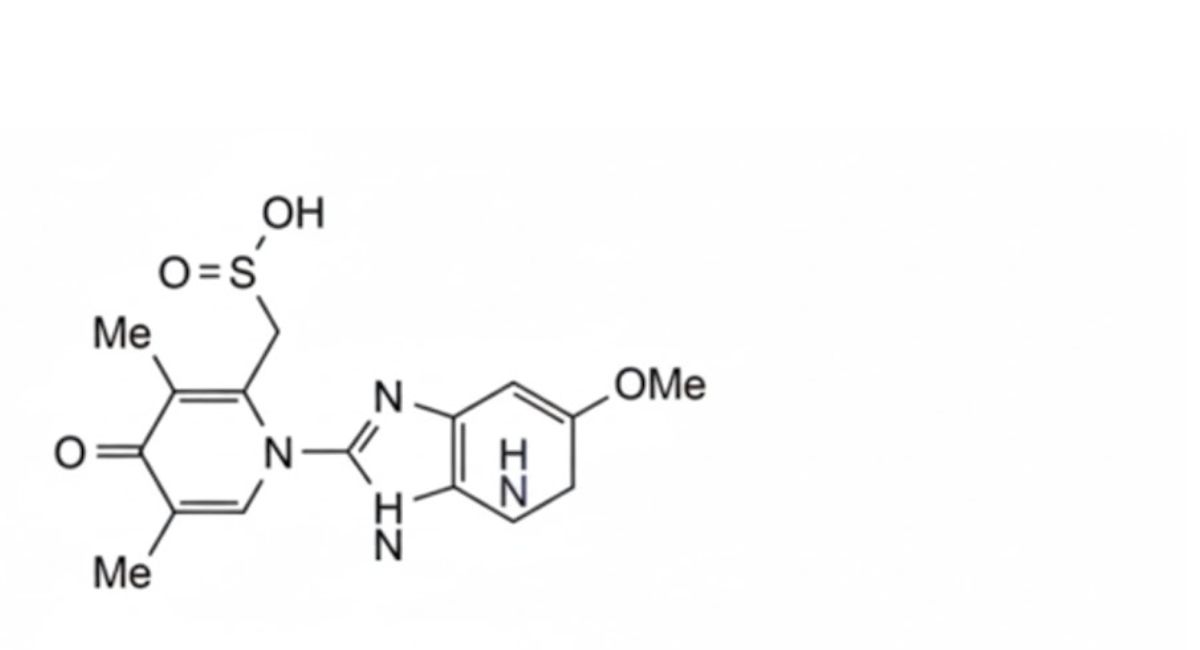
4. [1-(5-methoxy-1H-benzimidazol-2-yl)-3,5-dimethyl-4-oxo-1,4-dihydropyridin-2-yl] methanesulfinic acid.






