(Ph. Eur. monograph 0552)
Action and use
Macrolide antibacterial.
Preparation
Erythromycin Estolate Capsules
DEFINITION
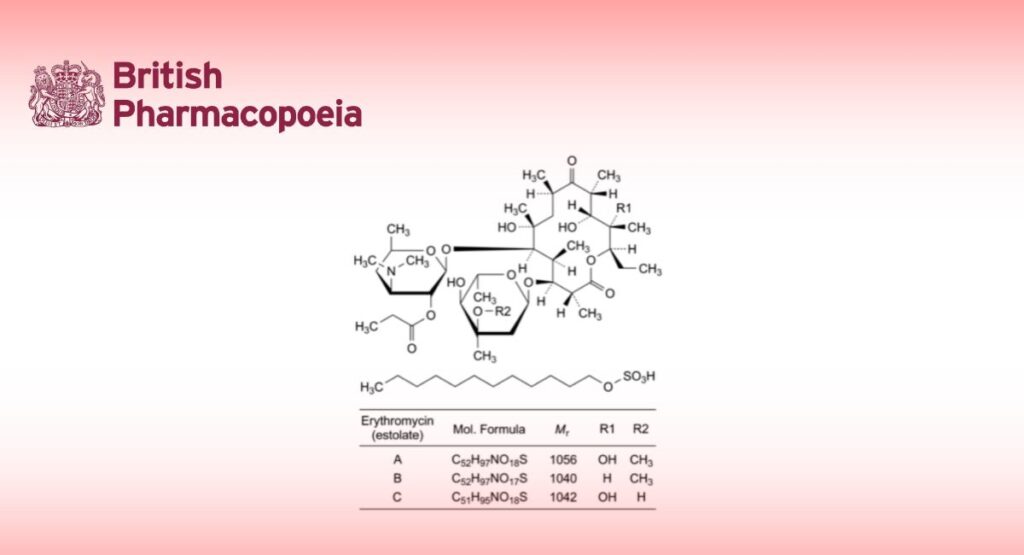
Mixture of the estolate esters of erythromycin.
Main component (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-
hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-2-O-propanyl-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione dodecyl sulfate (erythromycin A 2′′-propionate dodecyl sulfate).
Semi-synthetic product derived from a fermentation product obtained using a strain of Streptomycines erythreus.
Content
— sum of erythromycins A, B and C expressed as estolates: 93.0 per cent to 102.0 per cent (anhydrous substance);
— erythromycin B estolate: maximum 5.0 per cent (anhydrous substance);
— erythromycin C estolate: maximum 5.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, freely soluble in ethanol (96 per cent), soluble in acetone. It is practically insoluble in dilute hydrochloric acid.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison erythromycin estolate CRS.
TESTS
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use (apart from the test solution).
Solution A (hydrolysis solution). Dissolve 11.5 g of dipotassium hydrogen phosphate R in 900 mL of water R, adjust to pH 8.0 with dilute phosphoric acid R and dilute to 1000 mL with water R.
Test solution: Dissolve 0.150 g of the substance to be examined in 25 mL of methanol R. Add 20 mL of solution A, mix and allow to stand at room temperature for at least 12 h. Dilute to 50.0 mL with solution A.
Reference solution (a): Dissolve 40.0 mg of erythromycin A CRS in 12.0 mL of methanol R and dilute to 20.0 mL with solution A.
Reference solution (b): Dissolve 10.0 mg of erythromycin B CRS and 10.0 mg of erythromycin C CRS in 40.0 mL of methanol R and dilute to 100.0 mL with solution A.
Reference solution (c): Dilute 1.0 mL of reference solution (a) to 100.0 mL with a mixture of 4 volumes of solution A and 6 volumes of methanol R.
Reference solution (d) Dissolve 4 mg of erythromycin for system suitability CRS (containing impurities A, B, C, D, E, F, H and L) in 0.4 mL of methanol R and add 0.6 mL of solution A.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped polar-embedded octadecylsilyl amorphous organosilica polymer R (3.5 μm);
— temperature: 65 °C; preheating the mobile phase may be required, for instance by extending the inlet tubing in the oven to 30 cm.
Mobile phase:
— mobile phase A: phosphate buffer solution pH 7.0 R7, acetonitrile R1, water for chromatography R (5:35:60 V/V/V);
— mobile phase B: phosphate buffer solution ph 7.0 R7, water for chromatography R, acetonitrile R1 (5:45:50 V/V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – tR | 100 | 0 |
| tR- (tR+ 2) | 100 → 0 | 0 → 100 |
| (tR+ 2) – (tR+ 15) | 0 | 100 |
tR = retention time of erythromycin B, determined by injecting 20 μL of reference solution (b) and eluting with mobile phase A
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 210 nm.
Autosampler Set at 4 °C.
Injection 200 μL.
Identification of impurities Use the chromatogram supplied with erythromycin for system suitability CRS and the chromatogram obtained with reference solution (d) to identify the peaks due to impurities A, B, C, D, E, F and L; use the chromatogram obtained with reference solution (b) to identify the peaks due to erythromycins B and C.
Relative retention With reference to erythromycin A (retention time = about 23 min): impurity A = about 0.4;
impurity B = about 0.5; erythromycin C = about 0.55; impurity L = about 0.63; impurity C = about 0.9;
impurity D = about 1.61; erythromycin B = about 1.75; impurity F = about 1.81; impurity E = about 2.3.
System suitability Reference solution (d):
— resolution: minimum 1.2 between the peaks due to impurity B and erythromycin C;
— peak-to-valley ratio: minimum 1.5, where Hp = height above the baseline of the peak due to impurity F and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to erythromycin B; minimum 2.0, where Hp = height above the baseline of the peak due to impurity C and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to erythromycin A. If necessary, adjust the concentration of acetonitrile R1 in the mobile phases and/or the gradient to obtain the required separation.
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity D = 2; impurity E = 0.08; impurity F = 0.08; impurity L = 0.11;
— for each impurity, use the concentration of erythromycin A in reference solution (c).
Limits:
— impurity C: maximum 3.0 per cent;
— impurities A, B: for each impurity, maximum 2.0 per cent;
— impurities D, F: for each impurity, maximum 1.0 per cent;
— impurities eluting with relative retention between 2.1 and 2.4: not more than 4 impurities; for 1 impurity, maximum 3.5 per cent; for each other impurity, maximum 1.0 per cent;
— impurity L: maximum 0.4 per cent;
— any other impurity: for each impurity, maximum 0.4 per cent;
— total: maximum 7.0 per cent;
— reporting threshold: 0.2 per cent; disregard the peaks due to erythromycins B and C and any peak with a relative retention to erythromycin A of less than 0.3 (hydrolysis peaks).
Free erythromycin
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution: Dissolve 0.250 g of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution: Dissolve 75.0 mg of erythromycin A CRS in the mobile phase and dilute to 50.0 mL with the mobile phase. Dilute 5.0 mL of the solution to 25.0 mL with acetonitrile R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase: Mix 35 volumes of acetonitrile R1 and 65 volumes of a solution containing 3.4 g/L of potassium dihydrogen phosphate R and 2.0 g/L of triethylamine R, previously adjusted to pH 3.0 with dilute phosphoric acid R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 195 nm.
Injection 20 μL.
Run time: Twice the retention time of erythromycin A for the reference solution and 4.5 times the retention time of the 1 peak of erythromycin propionate for the test solution.
Retention time Erythromycin A = about 5 min; 1 peak of erythromycin propionate = about 10 min.
Limit:
— free erythromycin: not more than the area of the principal peak in the chromatogram obtained with the reference solution (6.0 per cent).
Dodecyl sulfate
23.0 per cent to 25.5 per cent of C12H26O4S (anhydrous substance).
Dissolve 0.500 g in 25 mL of dimethylformamide R. Titrate with 0.1 M sodium methoxide using 0.05 mL of a 3 g/L solution of thymol blue R in methanol R as indicator.
1 mL of 0.1 M sodium methoxide is equivalent to 26.64 mg of C12H26O4S.
Water (2.5.12)
Maximum 4.0 per cent, determined on 0.300 g.
Use a 100 g/L solution of imidazole R in anhydrous methanol R as the solvent.
Sulfated ash (2.4.14)
Maximum 0.5 per cent, determined on 0.5 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection Test solution and reference solutions (a) and (b).
System suitability Reference solution (a):
— symmetry factor: maximum 2.5 for the peak due to erythromycin A;
— repeatability: maximum relative standard deviation of 1.0 per cent determined on 6 injections.
Calculate the percentage content of erythromycin A (C37H67NO13) using the chromatogram obtained with reference solution (a). Calculate the percentage contents of erythromycin B (C37H67NO12) and erythromycin C (C36H65NO13) using the chromatogram obtained with reference solution (b). Express the result as erythromycin A estolate, erythromycin B estolate and erythromycin C estolate by multiplying the percentage content of erythromycin A by 1.4387, the percentage content of erythromycin B by 1.4485 and the percentage content of erythromycin C by 1.4472.
For the calculation of content of erythromycin estolate, use the sum of erythromycins A, B and C expressed as estolates as described above.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D, E, F, L.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) G, H, I, J, K, M, N.
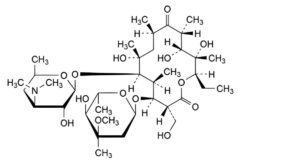
A. (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3-(hydroxymethyl)-5,7,9,11,13-pentamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (erythromycin F),
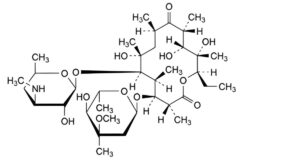
B. (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(methylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (3′′-N-demethylerythromycin A),
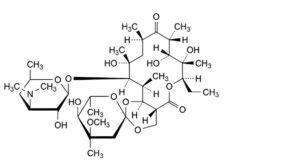
C. (2S,4aR,4′R,5′S,6′S,7R,8S,9R,10R,12R,14R,15R,16S,16aS)-7-ethyl-5′,8,9,14-tetrahydroxy-4′-methoxy-
4′,6′,8,10,12,14,16-heptamethyl-15-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-
hexopyranosyl]oxy]hexadecahydrospiro[5H,11H-1,3-dioxino[5,4-c]oxacyclotetradecin-2,2′-pyrane]-5,11-dione
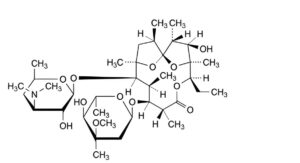
D. (1S,2R,3R,4S,5R,8R,9S,10S,11R,12R,14R)-9-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-5-ethyl-3-hydroxy-2,4,8,10,12,14-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-6,15,16-trioxatricyclo[10.2.1.1 ]hexadecan-7-one (anhydroerythromycin A),
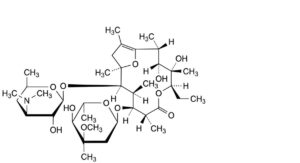
E. (2R,3R,4S,5R,8R,9S,10S,11R,12R)-9-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-5-ethyl-3,4-dihydroxy-2,4,8,10,12,14-hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-6,15-dioxabicyclo[10.2.1]pentadec-1(14)-en-7-one (erythromycin A enol ether),
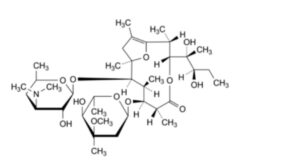
F. (2R,3R,6R,7S,8S,9R,10R)-7-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-3-[(1R,2R)-1,2-dihydroxy-1-methylbutyl]-2,6,8,10,12-pentamethyl-9-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-4,13-dioxabicyclo[8.2.1]tridec-1(12)-en-5-one (pseudoerythromycin A enol ether),
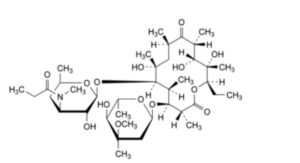
G. (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(methylpropanoylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (3′′-N-demethyl-3′′-N-propanoylerythromycin A),
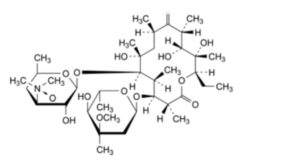
H. (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione N-oxide (erythromycin A 3′′-N-oxide),
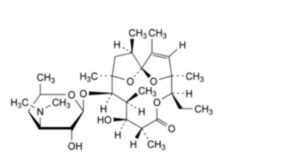
I. (1S,4S,5R,8R,9S,10S,11R,12R,14R)-5-ethyl-9-hydroxy-2,4,8,10,12,14-hexamethyl-11-[[3,4,6-trideoxy-3-
(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-6,15,16-trioxatricyclo[10.2.1.1 ]hexadec-2-en-7-one (erythralosamine), 1,4
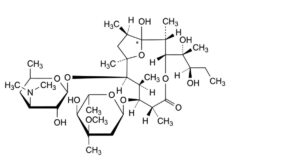
J. (1RS,2R,3R,6R,7S,8S,9R,10R,12R)-7-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-3-[(1R,2R)-1,2-dihydroxy-1-methylbutyl]-1-hydroxy-2,6,8,10,12-pentamethyl-9-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-4,13-dioxabicyclo[8.2.1]tridecan-5-one (pseudoerythromycin A hemiketal),
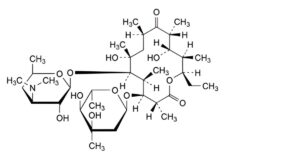
K. (3R,4S,5S,6R,7R,9R,11R,12S,13R,14R)-4-[(2,6-dideoxy-3-C-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12-dihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-
hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (erythromycin D),
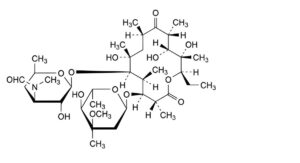
L. (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(formylmethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (3′′-N-demethyl-3′′-N-formylerythromycin A),
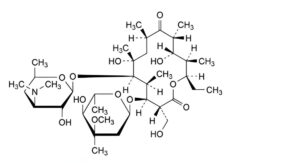
M. (3R,4S,5S,6R,7R,9R,11R,12S,13R,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12-dihydroxy-3-(hydroxymethyl)-5,7,9,11,13-pentamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione (erythromycin G),
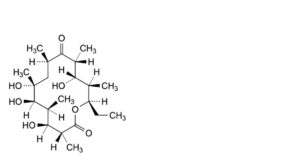
N. (3R,4S,5S,6R,7R,9R,11R,12S,13R,14R)-14-ethyl-4,6,7,12-tetrahydroxy-3,5,7,9,11,13-
hexamethyloxacyclotetradecane-2,10-dione (erythronolide B).