(Ph. Eur. monograph 1803)
C4H10O4 122.1 149-32-6
Action and use
Excipient.
DEFINITION
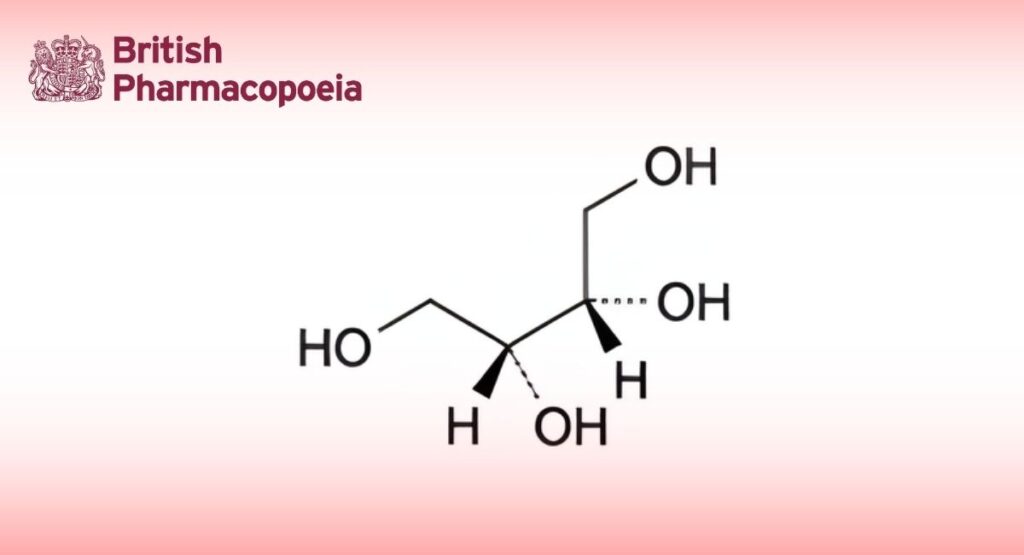
(2R,3S)-Butane-1,2,3,4-tetrol (meso-erythritol).
Content
96.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or free-flowing granules.
Solubility
Freely soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Melting point (2.2.14): 119 °C to 122 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: erythritol CRS.
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 5.0 g in water R and dilute to 50 mL with the same solvent.
Conductivity (2.2.38)
Maximum 20 μS·cm .
Dissolve 20.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution, while gently stirring with a magnetic stirrer.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.50 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 0.50 g of erythritol CRS in water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 2.0 mL of the test solution to 100.0 mL with water R.
Reference solution (c): Dilute 5.0 mL of reference solution (b) to 100.0 mL with water R.Reference solution (d) Dissolve 1.0 g of erythritol R and 1.0 g of glycerol R in water R and dilute to 20 mL with the same solvent.
Column:
— size: l = 0.3 m, Ø = 8.0 mm;
— stationary phase: cation-exchange resin R (9 μm);
— temperature: 70 °C.
Mobile phase 0.01 per cent V/V solution of sulfuric acid R.
Flow rate: 0.8 mL/min.
Detection: Differential refractometer maintained at a constant temperature (e.g. 35 °C).
Injection: 20 μL of the test solution and reference solutions (b), (c) and (d).
Run time: 3 times the retention time of erythritol.
Relative retention: With reference to erythritol (retention time = about 11 min): impurity A = about 0.77; impurity B = about 0.90; impurity C = about 0.94; impurity D = about 1.10.
System suitability: Reference solution (d):
— resolution: minimum 2.0 between the peaks due to erythritol and impurity D.
Limits:
— any impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2.0 per cent);
— total: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2.0 per cent);
— disregard limit: area of the principal peak in the chromatogram obtained with reference solution (c) (0.1 per cent).
Water (2.5.12)
Maximum 0.5 per cent, determined on 1.00 g.
Microbial contamination
If intended for use in the manufacture of parenteral preparations:
— TAMC: acceptance criterion 10 CFU/g (2.6.12).
If not intended for use in the manufacture of parenteral preparations:
— TAMC: acceptance criterion 10 CFU/g (2.6.12);
— TYMC: acceptance criterion 10 CFU/g (2.6.12);
— absence of Escherichia coli (2.6.13);
— absence of Salmonella (2.6.13).
Bacterial endotoxins (2.6.14)
If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins:
— less than 4 IU/g for parenteral preparations having a concentration of 100 g/L or less of erythritol;
— less than 2.5 IU/g for parenteral preparations having a concentration of more than 100 g/L of erythritol.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution and reference solution (a).
Calculate the percentage content of C4H10O4 taking into account the assigned content of erythritol CRS.
LABELLING
The label states where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
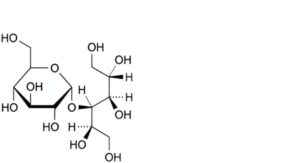
A. 4-O-α-D-glucopyranosyl-D-glucitol (D-maltitol),
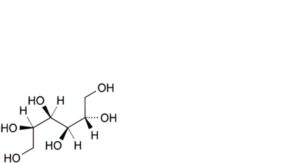
B. D-glucitol (D-sorbitol),
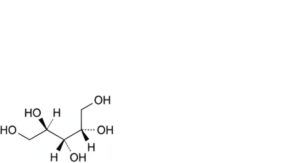
C. (2R,3s,4S)-pentane-1,2,3,4,5-pentol (meso-ribitol),
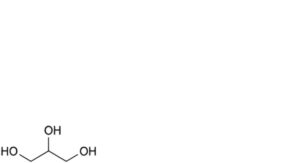
D. propane-1,2,3-triol (glycerol).