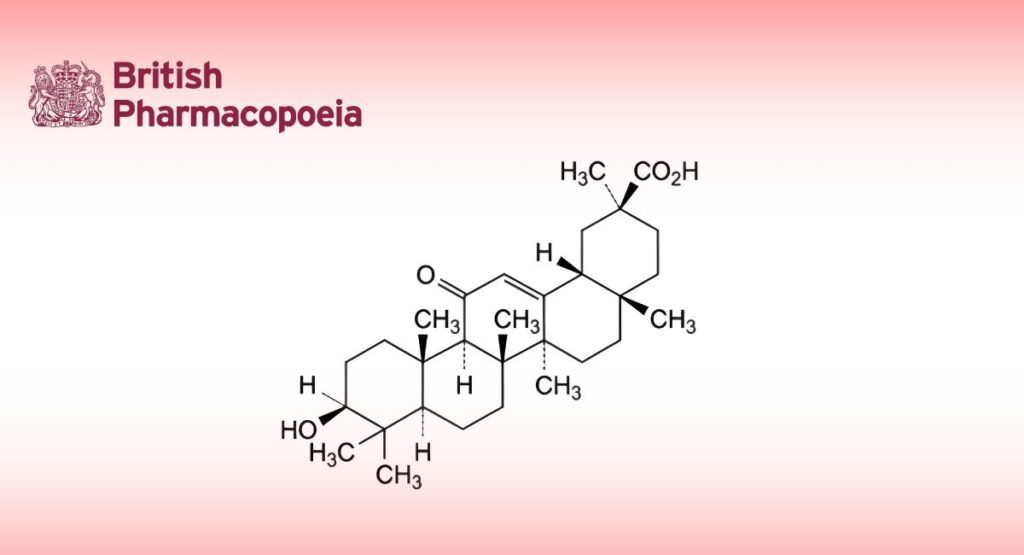
(Ph. Eur. monograph 1511)
C30H46O4 470.7 471-53-4
Action and use
Treatment of benign peptic ulcer disease.
DEFINITION
3β-Hydroxy-11-oxoolean-12-en-30-oic acid (glycyrrhetinic acid).
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white crystalline powder.
Solubility
Practically insoluble in water, soluble in anhydrous ethanol, sparingly soluble in methylene chloride.
It shows polymorphism (5.9).
IDENTIFICATION
First identification: A.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison enoxolone CRS.
If the spectra obtained in the solid state show differences, dissolve 0.2 g of the substance to be examined and 0.2 g of the reference substance separately in 6 mL of anhydrous ethanol R. Boil under a reflux condenser for 1 h and add 6 mL of water R. A precipitate is formed. Cool to about 10 °C and filter with the aid of vacuum. Wash the precipitate with 10 mL of ethanol (96 per cent) R, dry in an oven at 80 °C and record new spectra.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methylene chloride R and dilute to 10 mL with the same solvent.
Reference solution: Dissolve 10 mg of enoxolone CRS in methylene chloride R and dilute to 10 mL with the same solvent.
Plate TLC silica gel plate R.
Mobile phase glacial acetic acid R, acetone R, methylene chloride R (5:10:90 V/V/V).
Application 5 μL.
Development Over 2/3 of the plate.
Drying In air for 5 min.
Detection: Spray with anisaldehyde solution R and heat at 100-105 °C for 10 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Dissolve 50 mg in 10 mL of methylene chloride R. To 2 mL of this solution, add 1 mL of acetic anhydride R and 0.3 mL of sulfuric acid R. A pink colour is produced.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Dissolve 0.1 g in anhydrous ethanol R and dilute to 10 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 145 to + 154 (dried substance).
Dissolve 0.50 g in dioxan R and dilute to 50.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.10 g of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 5 mg of enoxolone impurity A CRS in tetrahydrofuran R and dilute to 5 mL with the same solvent. To 2.0 mL of the solution, add 2.0 mL of the test solution and dilute to 100.0 mL with the mobile phase.
Reference solution (c): Dissolve 5 mg of enoxolone for peak identification CRS (containing impurities B and C) in 2 mL of tetrahydrofuran R using sonication, and dilute to 5 mL with a 1.36 g/L solution of sodium acetate R adjusted to pH 4.8 with glacial acetic acid R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase: Mix 430 volumes of tetrahydrofuran R and 570 volumes of a 1.36 g/L solution of sodium acetate R adjusted to pH 4.8 with glacial acetic acid R.
Flow rate 0.8 mL/min.
Detection Spectrophotometer at 250 nm.
Injection 20 μL.
Run time 2 times the retention time of enoxolone.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity A; use the chromatogram supplied with enoxolone for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities B and C.
Relative retention With reference to enoxolone (retention time = about 21.5 min): impurity B = about 0.5;
impurity A = about 0.9; impurity C = about 1.3.
System suitability:
— resolution: minimum of 2.0 between the peaks due to impurity A and enoxolone in the chromatogram obtained with reference solution (b).
Calculation of percentage contents:
— for each impurity, use the concentration of enoxolone in reference solution (a).
Limits:
— impurity B: maximum 0.7 per cent;
— impurities A and C: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 1.0 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 4 h.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.330 g in 40 mL of dimethylformamide R. Titrate with 0.1 M tetrabutylammonium hydroxide, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
1 mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 47.07 mg of C30H46O4.
STORAGE
Protected from light.
IMPURITIES
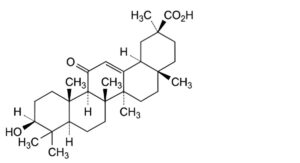
A. 3β-hydroxy-11-oxo-18α-olean-12-en-30-oic acid (18α-glycyrrhetinic acid),
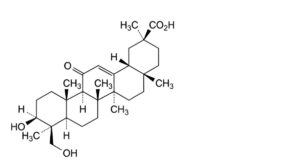
B. 3β,24-dihydroxy-11-oxoolean-12-en-30-oic acid,
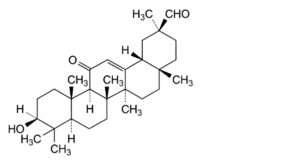
C. 3β-hydroxy-11-oxoolean-12-en-30-al (glycyrrhetaldehyde).