(Ph. Eur. monograph 1097)
Action and use
Low molecular weight heparin.
Preparation
Enoxaparin Sodium Injection
DEFINITION
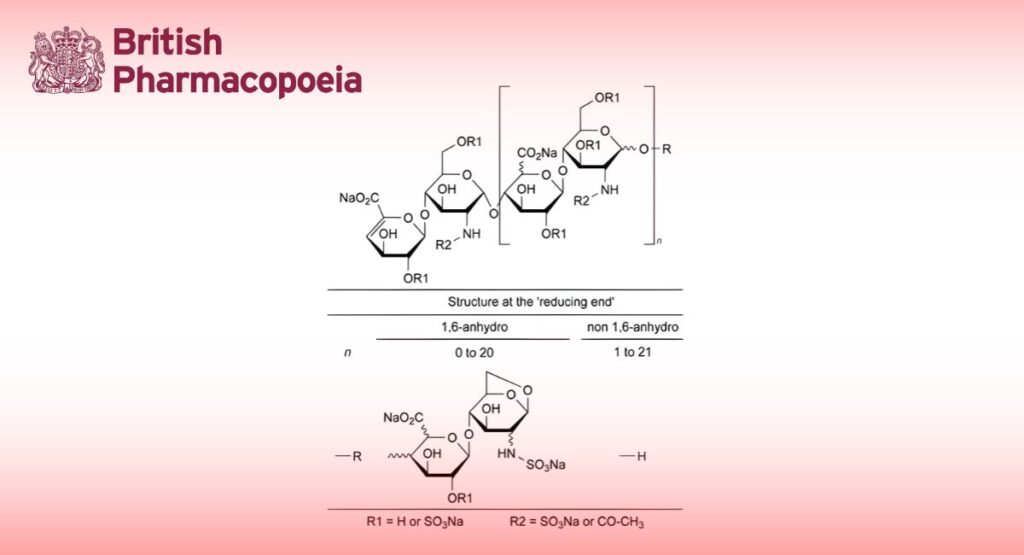
Enoxaparin sodium is the sodium salt of a low-molecular-mass heparin that is obtained by alkaline depolymerisation of the benzyl ester derivative of heparin from porcine intestinal mucosa. Enoxaparin consists of a complex set of oligosaccharides that have not yet been completely characterised. Based on current knowledge, the majority of the components have a 4-enopyranose uronate structure at the non-reducing end of their chain. 15 per cent to 25 per cent of the components have a 1,6-anhydro structure at the reducing end of their chain.
Enoxaparin sodium complies with the monograph Low-molecular-mass heparins (0828) with the modifications and
additional requirements below.
The mass-average relative molecular mass ranges between 3800 and 5000, with a characteristic value of about 4500.
The degree of sulfatation is about 2 per disaccharide unit.
The potency is not less than 90 IU and not more than 125 IU of anti-factor Xa activity per milligram, calculated with reference to the dried substance. The anti-factor IIa activity is not less than 20.0 IU and not more than 35.0 IU per milligram, calculated with reference to the dried substance. The ratio of anti-factor Xa activity to anti-factor IIa activity is between 3.3 and 5.3.
PRODUCTION
Enoxaparin is produced by alkaline depolymerisation of benzyl ester derivatives of heparin from porcine intestinal mucosa under conditions that yield a product complying with the structural requirements stated under Definition.
IDENTIFICATION
A. Carry out identification test A as described in the monograph Low-molecular-mass heparins (0828) using enoxaparin sodium CRS.
B. Liquid chromatography (2.2.29): use the normalisation procedure.
Solution A: Dissolve 12 mg of sodium tetrahydroborate R in 400 μL of water R and mix using a vortex mixer.
Heparinase solution (a) Dissolve heparinase I R in potassium phosphate buffer solution pH 7.0 R to obtain an activity of 0.4 IU/mL. Store the solution at – 20 °C until use.
Heparinase solution (b): Dissolve heparinase II R in potassium phosphate buffer solution pH 7.0 to obtain an activity of 0.4 IU/mL. Store the solution at – 20 °C until use.
Heparinase solution (c): Dissolve heparinase III R in potassium phosphate buffer solution pH 7.0 R to obtain an activity of 0.4 IU/mL. Store the solution at – 20 °C until use.
Heparinase solution (d): Mix equal volumes of heparinase solution (a), heparinase solution (b) and heparinase
solution (c).
Blank solution: Gently mix by inversion 20 μL of water R, 70 μL of sodium/calcium acetate buffer solution pH 7.0 R and 100 μL of heparinase solution (d). Place in a water-bath at 25 °C for 48 h. Mix 60 μL of this solution and 10 μL of freshly prepared solution A. Mix and allow to stand at room temperature for 4 h.
Test solution (a): Dissolve 20 mg of the substance to be examined in 1 mL of water R.
Test solution (b): To 20 μL of test solution (a), add 70 μL of sodium/calcium acetate buffer solution pH 7.0 R and 100 μL of heparinase solution (d). Gently mix by inversion and place in a water-bath at 25 °C for 48 h.
Test solution (c): To 60 μL of test solution (b), add 10 μL of freshly prepared solution A. Mix and allow to stand at room temperature for 4 h.
Reference solution (a): Dissolve 20 mg of enoxaparin sodium CRS in 1 mL of water R.
Reference solution (b): To 20 μL of reference solution (a), add 70 μL of sodium/calcium acetate buffer solution pH 7.0 R and 100 μL of heparinase solution (d). Gently mix by inversion and place in a water-bath at 25 °C for 48 h.
Reference solution (c): To 60 μL of reference solution (b), add 10 μL of freshly prepared solution A. Mix and allow to stand at room temperature for 4 h.
NOTE: heparinase solutions (a), (b) and (c) can be stored for 3 months at – 20 °C. Test solutions (a) and (b) and reference solutions (a) and (b) must be prepared at the same time; depolymerised test solutions are stable for 1 month at – 20 °C. Test solution (c) and reference solution (c) must also be prepared at the same time.
Precolumn:
— size: l = 0.01 m, Ø = 4.6 mm;
— stationary phase: strongly basic anion-exchange resin for chromatography R (5 μm).
Column:
— size: l = 0.25 m, Ø = 4 mm;
— stationary phase: strongly basic anion-exchange resin for chromatography R (5 μm);
— temperature: 50 °C.
Mobile phase:
— mobile phase A: dissolve 0.280 g of sodium dihydrogen phosphate R in 950 mL of water R, adjust to pH 3.0 with phosphoric acid R and dilute to 1000 mL with water R;
— mobile phase B: dissolve 140 g of sodium perchlorate R in 950 mL of mobile phase A, adjust to pH 3.0 with phosphoric acid R and dilute to 1000 mL with mobile phase A;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 20 | 97 → 65 | 3 → 35 |
| 20 – 50 | 65 → 0 | 35 → 100 |
| 50 – 60 | 0 | 100 |
Flow rate: 0.8 mL/min.
Detection: Spectrophotometer at 234 nm.
Injection: 18 μL of the blank solution, test solution (c) and reference solutions (b) and (c).
Identification of disaccharides: Use the chromatogram supplied with enoxaparin sodium CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to the disaccharides listed in Table 1097.-1; use the chromatogram obtained with reference solution (b) to confirm the identity of the peaks due to the 1,6-anhydro derivatives.
Table 1097.-1. – Correlation between the relative retention of peaks observed in chromatograms obtained with solutions of depolymerised and reduced enoxaparin, with reference to reduced ∆IS (retention time = about 30 min), and molecular masses of the enoxaparin derivatives
| Derivatives | Relative retention | Molecular mass (Da) |
| Unidentified | < 0.20 | 741 |
| Reduced ΔIVA | 0.20 | 401 |
| Unidentified | 0.20 – 0.46 | 741 |
| Reduced ΔIVS | 0.46 | 461 |
| Unidentified | 0.46 – 0.48 | 483 |
| Reduced ΔIIA | 0.48 | 503 |
| Unidentified | 0.48 – 0.52 | 503 |
| 1,6-anhydro ΔIIS | 0.52 | 443 |
| Unidentified | 0.52 – 0.57 | 503 |
| Reduced ΔIIIA | 0.57 | 503 |
| Unidentified | 0.57 – 0.66 | 533 |
| Reduced ΔIIS | 0.66 | 563 |
| Unidentified | 0.66 – 0.76 | 563 |
| Reduced ΔIIIS | 0.76 | 563 |
| Unidentified | 0.76 – 0.85 | 583 |
| Reduced ΔIA | 0.85 | 605 |
| 1,6-anhydro ΔIS | 0.88 | 545 |
| Unidentified | 0.88 – 0.97 | 635 |
| Reduced ΔIIA-IVSglu | 0.97 | 1066 |
| Reduced ΔIS | 1.00 | 665 |
| ΔIS | 1.04 | 665 |
| Unidentified | 1.04 – 1.10 | 1228 |
| Reduced ΔIIA-IISglu | 1.10 | 1168 |
| Unidentified | 1.10 – 1.28 | 1228 |
| 1,6-anhydro ΔIS-IS | 1.28 | 1210 |
| Unidentified | > 1.28 | 1228 |
NOTE: depending on the resolution of the column, 1,6-anhydro ∆IIS may be eluted in the form of 2 peaks (mannosamine and glucosamine forms), which are both taken into account as 1,6-anhydro ∆IIS.
Relative retention: With reference to reduced ∆IS (retention time = about 30 min): see Table 1097.-1.
System suitability:
— peak area ratio: maximum 1.15 for the peaks due to 1,6-anhydro ∆IS-IS and 1,6-anhydro ∆IS in the chromatogram obtained with reference solution (b); maximum 0.02 for the peaks due to ∆IS and reduced ∆IS in the chromatogram obtained with reference solution (c);
— resolution: minimum 1.5 between the peaks due to reduced ∆IA and 1,6-anhydro ∆IS in the chromatogram
obtained with reference solution (c);
— the content of 1,6-anhydro derivatives in enoxaparin sodium CRS is within 1.5 per cent of the assigned content.
Calculation:
Calculate the molar percentage of the 3 main 1,6-anhydro derivatives using the relative molecular masses given in
Table 1097.-1 and the following expression:
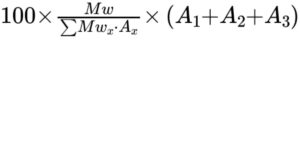
Mw = mass-average relative molecular mass of enoxaparin (as determined by identification test C);
Mwx = relative molecular mass attributed to derivative x according to Table 1097.-1;
Ax = area of the peak due to derivative x;
A1 = area of the peak due to 1,6-anhydro ΔIS;
A2 = area of the peak due to 1,6-anhydro ΔIIS;
A3 = area of the peak due to 1,6-anhydro ΔIS-IS.
Disregard any peak observed with the blank solution.
Correct the value to the nearest unit.
Limit: 15 per cent to 25 per cent of components bearing the 1,6-anhydro structure at the reducing end of their chain.
C. Carry out identification test C as described in the monograph Low-molecular-mass heparins (0828). The following requirements apply.
The mass-average relative molecular mass ranges between 3800 and 5000. The mass percentage of chains lower than 2000 ranges between 12.0 per cent and 20.0 per cent. The mass percentage of chains between 2000 and 8000 ranges between 68.0 per cent and 82.0 per cent.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than intensity 6 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
Dissolve 1.0 g in 10 mL of water R.
pH (2.2.3)
6.2 to 7.7.
Dissolve 1.0 g in carbon dioxide-free water R and dilute to 10.0 mL with the same solvent.
Specific absorbance (2.2.25)
14.0 to 20.0 (dried substance), determined at 231 nm.
Dissolve 50.0 mg in 100 mL of 0.01 M hydrochloric acid.
Benzyl alcohol
Liquid chromatography (2.2.29).
Internal standard solution: 1 g/L solution of 3,4-dimethylphenol R in methanol R.
Test solution: Dissolve about 0.500 g of the substance to be examined in 5.0 mL of 1 M sodium hydroxide. Allow to stand for 1 h. Add 1.0 mL of glacial acetic acid R and 1.0 mL of the internal standard solution and dilute to 10.0 mL with water R.
Reference solution: Prepare a 0.25 g/L solution of benzyl alcohol R in water R. Mix 0.50 mL of this solution with 1.0 mL of the internal standard solution and dilute to 10.0 mL with water R.
Precolumn:
— size: l = 0.02 m, Ø = 4.6 mm;
— stationary phase: octylsilyl silica gel for chromatography R (5 μm).
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: octylsilyl silica gel for chromatography R (5 μm).
Mobile phase: methanol R, acetonitrile R, water R (5:15:80 V/V/V).
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 256 nm.
Injection: 20 μL.
From the chromatogram obtained with the reference solution, calculate the ratio (R1) of the height of the peak due to benzyl alcohol to the height of the peak due to the internal standard. From the chromatogram obtained with the test solution, calculate the ratio (R2) of the height of the peak due to benzyl alcohol to the height of the peak due to the internal standard.
Calculate the percentage content m/m of benzyl alcohol using the following expression:
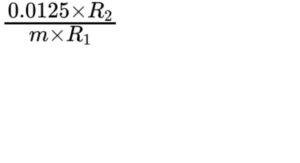
m = mass of the substance to be examined, in grams.
Limit:
— benzyl alcohol: maximum 0.1 per cent m/m.
Sodium (2.2.23, Method I)
11.3 per cent to 13.5 per cent (dried substance).