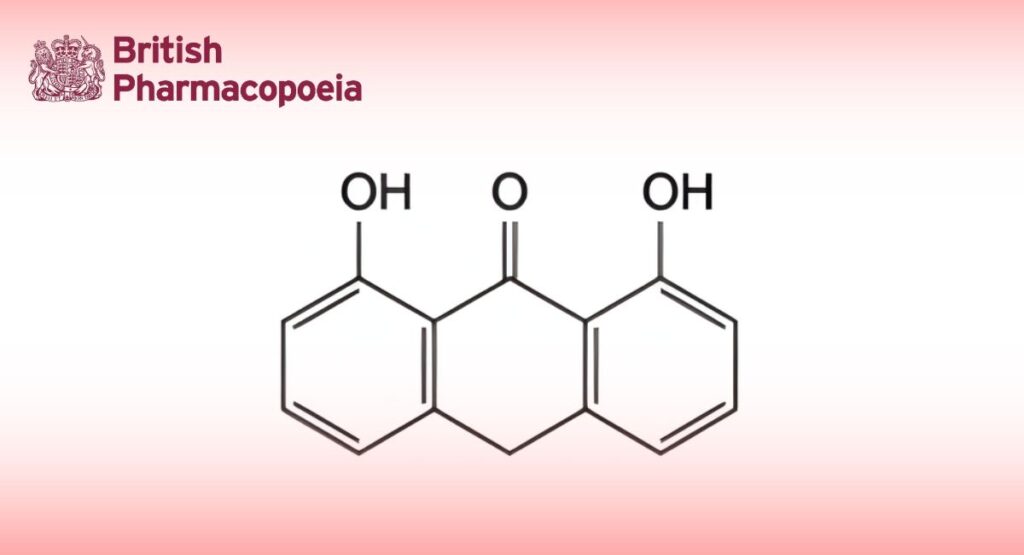
(Ph. Eur. monograph 1007)
C14H10O3 226.2 1143-38-0Action and use
Coal tar extract; treatment of psoriasis.
Preparations
Dithranol Cream
Dithranol Ointment
Dithranol Paste
Dithranol and Salicylic Acid Ointment
DEFINITION
1,8-Dihydroxyanthracen-9(10H)-one.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
Yellow or brownish-yellow, crystalline powder.
Solubility
Practically insoluble in water, soluble in methylene chloride, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides.
Carry out all tests protected from bright light and use freshly prepared solutions.
IDENTIFICATION
First identification: A, B.
Second identification: A, C, D.
A. Melting point (2.2.14): 178 °C to 182 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: dithranol CRS.
C. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methylene chloride R and dilute to 10 mL with the same solvent.
Reference solution (a): Dissolve 10 mg of dithranol CRS in methylene chloride R and dilute to 10 mL with the same solvent.
Reference solution (b): Dissolve about 5 mg of dantron R (impurity B) in 5 mL of reference solution (a).
Plate: TLC silica gel plate R.
Mobile phase hexane R, methylene chloride R (50:50 V/V).
Application: 10 μL.
Development: Over a path of 12 cm.
Drying: In air.
Detection: Place the plate in a tank saturated with ammonia vapour until the spots appear. Examine in daylight.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
D. To 5 mg add 0.1 g of anhydrous sodium acetate R and 1 mL of acetic anhydride R. Boil for 30 s. Add 20 mL of ethanol (96 per cent) R. Examined in ultraviolet light at 365 nm, the solution shows a blue fluorescence.
TESTS
Related substances
A. Liquid chromatography (2.2.29).
Test solution: Dissolve 0.200 g of the substance to be examined in 20 mL of methylene chloride R, add 1.0 mL of glacial acetic acid R and dilute to 100.0 mL with hexane R.
Reference solution: Dissolve 5.0 mg of anthrone R (impurity A), 5.0 mg of dantron R (impurity B), 5.0 mg of dithranol impurity C CRS and 5.0 mg of dithranol CRS in methylene chloride R and dilute to 5.0 mL with the same solvent. To 1.0 mL of this solution, add 19.0 mL of methylene chloride R and 1.0 mL of glacial acetic acid R, and dilute to 50.0 mL with hexane R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: silica gel for chromatography R (5 μm).
Mobile phase: glacial acetic acid R, methylene chloride R, hexane R (1:5:82 V/V/V).
Flow rate: 2 mL/min.
Detection: Spectrophotometer at 260 nm.
Injection: 20 μL.
Run time: 1.5 times the retention time of impurity C.
Elution order: Dithranol, impurity B, impurity A, impurity C.
System suitability: Reference solution:
— resolution: minimum 2.0 between the peaks due to dithranol and impurity B.
Limits:
— impurities A, B, C: for each impurity, not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (1 per cent).
B. Liquid chromatography (2.2.29).
Test solution: Dissolve 25.0 mg of the substance to be examined in 5 mL of tetrahydrofuran R and dilute to 25.0 mL with the mobile phase.
Reference solution: Dissolve 5.0 mg of dithranol impurity D CRS and 5.0 mg of dithranol CRS in 5 mL of tetrahydrofuran R and dilute to 10.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Column:
— size: l = 0.20 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: glacial acetic acid R, tetrahydrofuran R, water R (2.5:40:60 V/V/V).
Flow rate: 0.9 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 20 μL.
Run time: 3 times the retention time of dithranol.
System suitability: Reference solution:
— resolution: minimum 2.5 between the peaks due to impurity D and dithranol.
Limit:
— impurity D: not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (2.5 per cent).
Total (tests A + B): Maximum 3.0 per cent for the sum of the contents of all impurities.
Chlorides (2.4.4)
Maximum 100 ppm.
Shake 1.0 g with 20 mL of water R for 1 min and filter. Dilute 10 mL of the filtrate to 15 mL with water R.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g in 50 mL of anhydrous pyridine R. Titrate with 0.1 M tetrabutylammonium hydroxide under nitrogen R.
Determine the end-point potentiometrically (2.2.20), using a glass indicator electrode and a suitable reference electrode.
1 mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 22.62 mg of C14H10O3.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D.
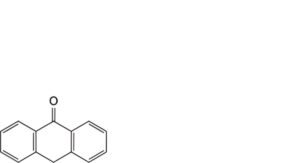
A. anthracen-9(10H)-one (anthrone),
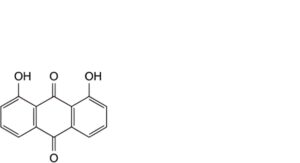
B. 1,8-dihydroxyanthracene-9,10-dione (dantron),
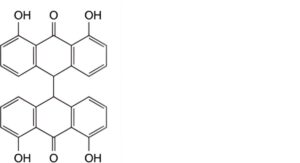
C. 4,4′,5,5′-tetrahydroxy-9,9′-bianthracenyl-10,10′(9H,9′H)-dione,
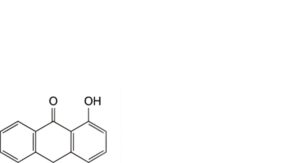
D. 1-hydroxyanthracen-9(10H)-one.