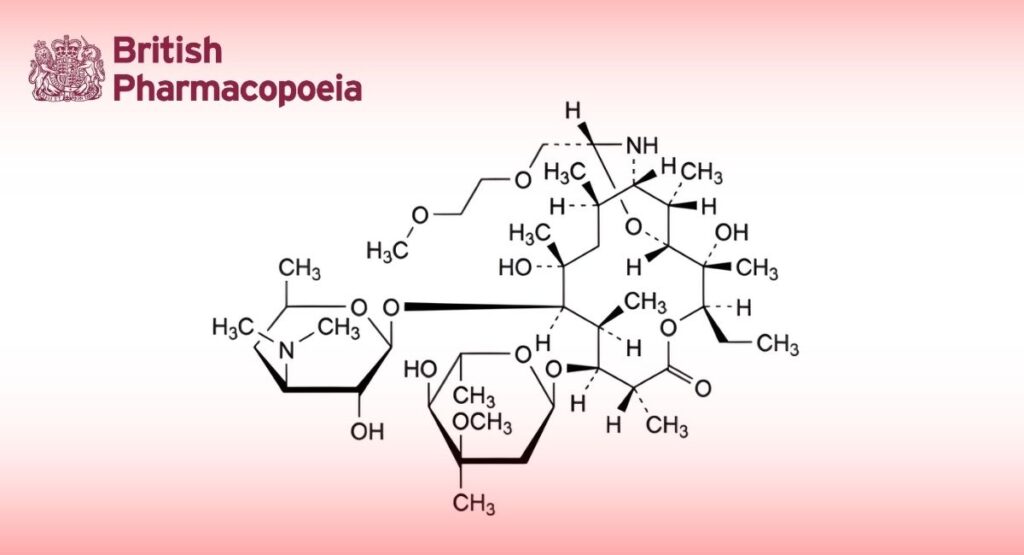
(Ph. Eur. monograph 1313)
C42H78N2O14 835 62013-04-1
Action and use
Macrolide antibacterial.
DEFINITION
(1R,2S,3R,6R,7S,8S,9R,10R,12R,13S,15R,17S)-9-[[3-(Dimethylamino)-3,4,6-trideoxy-β-D-xylo-hexopyranosyl]oxy]-3-ethyl-2,10-dihydroxy-15-[(2-methoxyethoxy)methyl]-2,6,8,10,12,17-hexamethyl-7-[(3-C-methyl-3-O-methyl-2,6dideoxy-α-L-ribo-hexopyranosyl)oxy]-4,16-dioxa-14azabicyclo[11.3.1]heptadecan-5-one (or (9S)-9,11-[imino[(1R)-2-(2-methoxyethoxy)ethylidene]oxy]-9-deoxo-11-deoxyerythromycin).
Semi-synthetic product derived from a fermentation product.
Content
96.0 per cent to 102.0 per cent for the sum of the percentage contents of C42H78N2O14 and dirithromycin 15S-epimer (anhydrous substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Very slightly soluble in water, very soluble in methanol and in methylene chloride.
It shows polymorphism (5.9).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: dirithromycin CRS.
B. Examine the chromatograms obtained in the assay.
Results: The principal peak in the chromatogram obtained with test solution (a) is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture methanol R, acetonitrile R1 (30:70 V/V).
Test solution (a): Dissolve 20.0 mg of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Test solution (b): Dissolve 0.10 g of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a): Dissolve 20.0 mg of dirithromycin CRS in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (b): Dilute 5.0 mL of reference solution (a) to 50.0 mL with the solvent mixture.
Reference solution (c): Dissolve 20 mg of dirithromycin CRS in the mobile phase and dilute to 10 mL with the mobile phase. Allow to stand for 24 h before use.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 40 °C.
Mobile phase: Mix 9 volumes of water R, 19 volumes of methanol R, 28 volumes of a solution containing 1.9 g/L of potassium dihydrogen phosphate R and 9.1 g/L of dipotassium hydrogen phosphate R adjusted to pH 7.5 if necessary with a 100 g/L solution of potassium hydroxide R, and 44 volumes of acetonitrile R1.
Flow rate: 2.0 mL/min.
Detection: Spectrophotometer at 205 nm.
Injection: 10 μL of test solution (b) and reference solutions (b) and (c).
Run time: 3 times the retention time of dirithromycin.
Relative retention: With reference to dirithromycin: impurity A = about 0.7; 15S-epimer = about 1.1.
System suitability: Reference solution (c):
— resolution: minimum 2.0 between the peaks due to dirithromycin and its 15S-epimer; if necessary, adjust the concentration of the organic modifiers in the mobile phase.
Limits:
— impurity A: not more than 0.75 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.5 per cent);
— any other impurity: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1 per cent);
— disregard limit: disregard the peak due to the 15S-epimer.
Dirithromycin 15S-epimer
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection: Test solution (b) and reference solution (b).
System suitability: Reference solution (b):
— repeatability: maximum relative standard deviation of 5.0 per cent after 6 injections.
Limit:
— 15S-epimer: maximum 1.5 per cent.
Acetonitrile (2.4.24, System A)
Maximum 0.1 per cent.
Prepare the solutions using dimethylformamide R instead of water R.
Sample solution: Dissolve 0.200 g of the substance to be examined in dimethylformamide R and dilute to 20.0 mL with the same solvent.
Static head-space injection conditions that may be used:
— equilibration temperature: 120 °C;
— equilibration time: 60 min;
— transfer-line temperature: 125 °C.
Water (2.5.12)
Maximum 1.0 per cent, determined on 1.00 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection: Test solution (a) and reference solution (a).
System suitability: Reference solution (a):
— repeatability: maximum relative standard deviation of 1.0 per cent after 6 injections.
IMPURITIES
Specified impurities A.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, D, E.
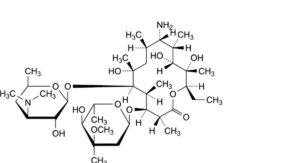
A. (9S)-9-amino-9-deoxoerythromycin,
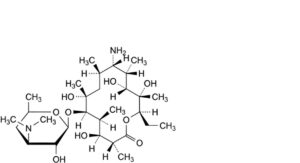
B. (9S)-9-amino-3-de(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)-9-deoxoerythromycin,
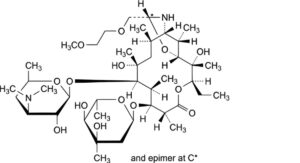
C. (9S)-9,11-[imino[(1RS)-2-(2-methoxyethoxy)ethylidene]oxy]-9-deoxo-11,12-dideoxyerythromycin (dirithromycin B)
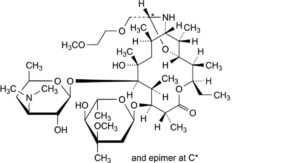
D. (9S)-9,11-[imino[(1RS)-2-(2-methoxyethoxy)ethylidene]oxy]-3′-O-demethyl-9-deoxo-11-deoxyerythromycin (dirithromycin C),
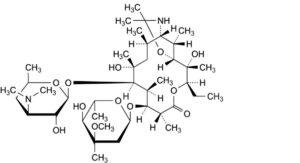
E. 9,11-[imino(1-methylethylidene)oxy]-9-deoxo-11-deoxyerythromycin.