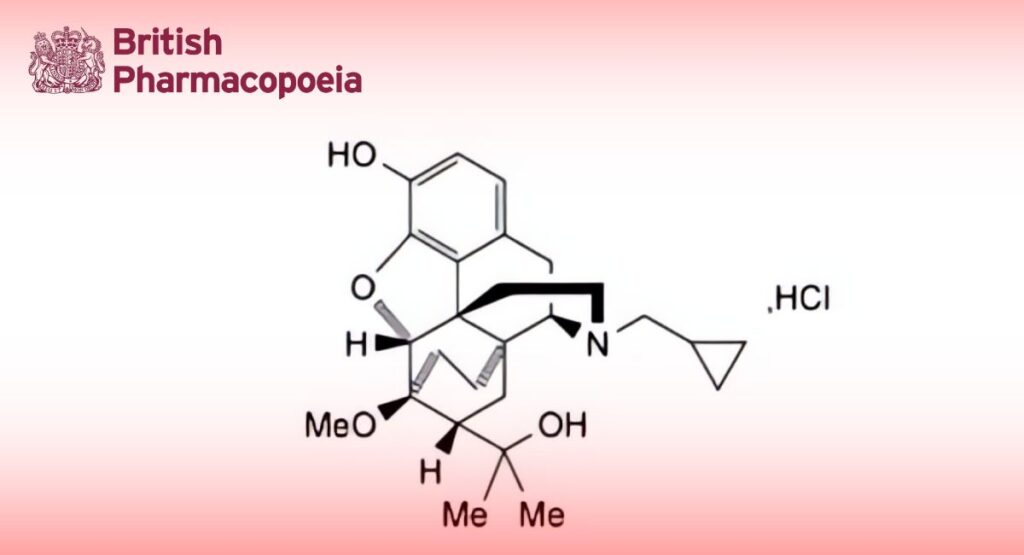
C26H35NO4,HCl 462.1 16808-86-9
Action and use
Opioid receptor antagonist.
Preparation
Diprenorphine Injection
DEFINITION
Diprenorphine Hydrochloride is N-cyclopropylmethyl-7,8-dihydro-7α-(1-hydroxy-1-methylethyl)-6-O-methyl-6α,14α- ethanonormorphine hydrochloride. It contains not less than 98.5% and not more than 101.0% of C26H35NO4,HCl , calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white, crystalline powder.
Sparingly soluble in water; slightly soluble in ethanol (96%); very slightly soluble in chloroform; practically insoluble in ether.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of diprenorphine hydrochloride (RSV 18).
B. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.02% w/v solution in 0.1M hydrochloric acid exhibits a maximum only at 287 nm. The absorbance at the maximum is about 0.70.
C. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.02% w/v solution in 0.1M sodium hydroxide exhibits a maximum only at 301 nm. The absorbance at the maximum is about 1.1.
D. Yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH of a 2.0% w/v solution, 4.5 to 6.0, Appendix V L.
Specific optical rotation
In a solution prepared by dissolving 0.5 g in sufficient methanol to produce 25 mL, -97.0 to -107.0, calculated with reference to the dried substance, Appendix V F.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using silica gel G as the coating substance and a mixture of 1 volume of water, 5 volumes of methanol, 30 volumes of butan-2-one, 80 volumes of acetone and 100 volumes of cyclohexane as the mobile phase. Apply separately to the plate 20 μL of each of two solutions of the substance being examined in methanol containing (1) 2.0% w/v and (2) 0.020% w/v. Add at each point of application 10 μL of a mixture of 4 volumes of methanol and 1 volume of 13.5M ammonia. After removal of the plate, allow it to dry in air and spray with a 1% w/v solution of iodine in methanol. Any secondary spot in the chromatogram obtained with solution (1) is not more intense
than the spot in the chromatogram obtained with solution (2) (1%).
Loss on drying
When dried to constant weight at 105°, loses not more than 2.0% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.5 g, adding 7 mL of mercury(II) acetate solution and using crystal violet solution as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 46.21 mg C26H35NO4,HCl.
STORAGE
Diprenorphine Hydrochloride should be protected from light.