(Ph. Eur. monograph 0389)
C3H8OS2 124.2 59-52-9
Action and use
Chelating agent for use in heavy metal poisoning.
Preparation
Dimercaprol Injection
When B.A.L. is prescribed or demanded, Dimercaprol shall be dispensed or supplied.
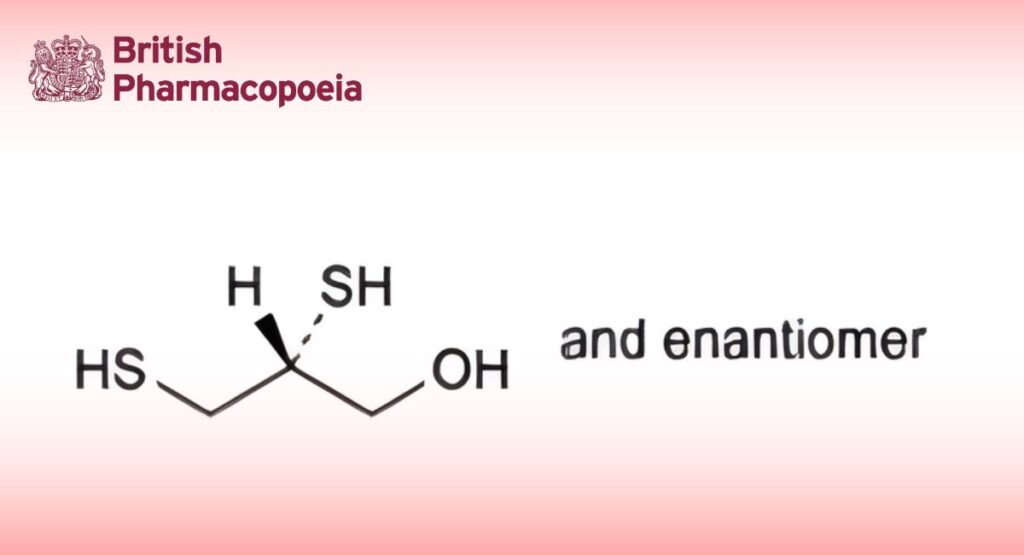
DEFINITION
(2RS)-2,3-Disulfanylpropan-1-ol.
Content
98.5 per cent to 101.5 per cent.
CHARACTERS
Appearance
Clear, colourless or slightly yellow liquid.
Solubility
Soluble in water and in arachis oil, miscible with ethanol (96 per cent) and with benzyl benzoate.
IDENTIFICATION
A. Dissolve 0.05 mL in 2 mL of water R. Add 1 mL of 0.05 M iodine. The colour of the iodine is discharged immediately.
B. Dissolve 0.1 mL in 5 mL of water R and add 2 mL of copper sulfate solution R. A bluish-black precipitate is formed which quickly becomes dark grey.
C. In a ground-glass-stoppered tube, suspend 0.6 g of sodium bismuthate R, previously heated to 200 °C for 2 h, in a mixture of 2.8 mL of dilute phosphoric acid R and 6 mL of water R. Add 0.2 mL of the substance to be examined, mix and allow to stand for 10 min with frequent shaking. To 1 mL of the supernatant add 5 mL of a 4 g/L solution of chromotropic acid, sodium salt R in sulfuric acid R and mix. Heat in a water-bath for 15 min. A violet-red colour develops.
TESTS
Appearance
It is clear (2.2.1) and not more intensely coloured than reference solution B6 or BY6 (2.2.2, Method II).
Acidity or alkalinity
Dissolve 0.2 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent. Add 0.25 mL of bromocresol green solution R and 0.3 mL of 0.01 M hydrochloric acid.
The solution is yellow. Not more than 0.5 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to blue.
Refractive index (2.2.6)
1.568 to 1.574.
Halides
To 2.0 g add 25 mL of alcoholic potassium hydroxide solution R and boil under a reflux condenser for 2 h. Eliminate the ethanol by evaporation in a stream of hot air. Add 20 mL of water R and cool. Add 40 mL of water R and 10 mL of strong hydrogen peroxide solution R, boil gently for 10 min, cool and filter rapidly. Add 10 mL of dilute nitric acid R and 5.0 mL of 0.1 M silver nitrate. Using 2 mL of ferric ammonium sulfate solution R2 as indicator, titrate with 0.1 M ammonium thiocyanate until a reddish-yellow colour is obtained. Carry out a blank titration. The difference between the titration volumes is not greater than 1.0 mL.
ASSAY
Dissolve 0.100 g in 40 mL of methanol R. Add 20 mL of 0.1 M hydrochloric acid and 50.0 mL of 0.05 M iodine. Allow to stand for 10 min and titrate with 0.1 M sodium thiosulfate. Carry out a blank titration.
1 mL of 0.05 M iodine is equivalent to 6.21 mg of C3H8OS2.
STORAGE
In a well-filled, airtight container, protected from light, at a temperature of 2 °C to 8 °C.