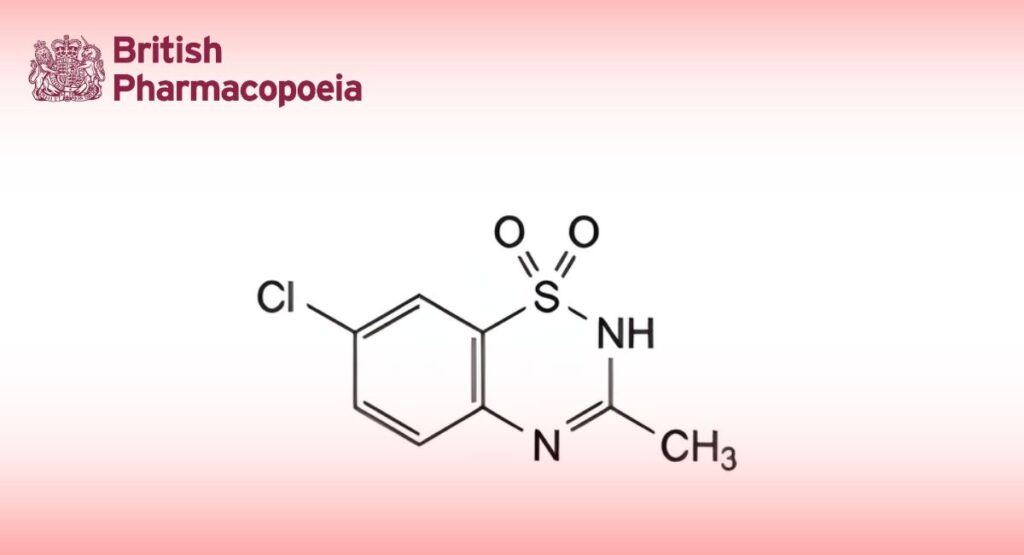
(Ph. Eur. monograph 0550)
C8H7ClN2O2S 230.7 364-98-7
Action and use
Vasodilator; Treatment of hypertension.
Preparations
Diazoxide Injection
Diazoxide Tablets
DEFINITION
Diazoxide contains not less than 98.0 per cent and not more than the equivalent of 101.0 per cent of 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide, calculated with reference to the dried substance.
CHARACTERS
A white or almost white, fine or crystalline powder, practically insoluble in water, freely soluble in dimethylformamide, slightly soluble in ethanol (96 per cent). It is very soluble in dilute solutions of the alkali hydroxides.
IDENTIFICATION
First identification: B.
Second identification: A, C, D.
A. Dissolve 50.0 mg in 5 mL of 1 M sodium hydroxide and dilute to 50.0 mL with water R. Dilute 1.0 mL of this solution to 100.0 mL with 0.1 M sodium hydroxide. Examined between 230 nm and 350 nm (2.2.25), the solution shows an absorption maximum at 280 nm and a shoulder at 304 nm. The specific absorbance at the maximum is 570 to 610.
B. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with diazoxide CRS. Examine the substances prepared as discs using potassium bromide R.
C. Examine the chromatograms obtained in the test for related substances in ultraviolet light at 254 nm. The principal spot in the chromatogram obtained with test solution (b) is similar in position and size to the principal spot in the chromatogram obtained with reference solution (b).
D. Dissolve about 20 mg in a mixture of 5 mL of hydrochloric acid R and 10 mL of water R. Add 0.1 g of zinc powder R.
Boil for 5 min, cool and filter. To the filtrate add 2 mL of a 1 g/L solution of sodium nitrite R and mix. Allow to stand for 1 min and add 1 mL of a 5 g/L solution of naphthylethylenediamine dihydrochloride R. A red or violet-red colour develops.
TESTS
Appearance of solution
Dissolve 0.4 g in 2 mL of 1 M sodium hydroxide and dilute to 20 mL with water R. The solution is clear (2.2.1) and not more intensely coloured than reference solution Y7
(2.2.2, Method II).
Acidity or alkalinity
To 0.5 g of the powdered substance to be examined add 30 mL of carbon dioxide-free water R, shake for 2 min and filter.
To 10 mL of the filtrate add 0.2 mL of 0.01 M sodium hydroxide and 0.15 mL of methyl red solution R. The solution is yellow. Not more than 0.4 mL of 0.01 M hydrochloric acid is required to change the colour of the indicator to red.
Related substances
Examine by thin-layer chromatography (2.2.27), using silica gel GF254 R as the coating substance.
Test solution (a): Dissolve 0.1 g of the substance to be examined in a mixture of 0.5 mL of 1 M sodium hydroxide and 1 mL of methanol R and dilute to 5 mL with methanol R.
Test solution (b): Dilute 1 mL of test solution (a) to 5 mL with a mixture of 1 volume of 1 M sodium hydroxide and 9 volumes of methanol R.
Reference solution (a): Dilute 0.5 mL of test solution (a) to 100 mL with a mixture of 1 volume of 1 M sodium hydroxide and 9 volumes of methanol R.
Reference solution (b): Dissolve 20 mg of diazoxide CRS in a mixture of 0.5 mL of 1 M sodium hydroxide and 1 mL of methanol R and dilute to 5 mL with methanol R.
Apply separately to the plate 5 μL of each solution. Develop over a path of 15 cm using a mixture of 7 volumes of concentrated ammonia R, 25 volumes of methanol R and 68 volumes of chloroform R. Allow the plate to dry in air and examine in ultraviolet light at 254 nm. Any spot in the chromatogram obtained with test solution (a), apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (a) (0.5 per cent).
Loss on drying (2.2.32)
Not more than 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g with gentle heating in 50 mL of a mixture of 1 volume of water R and 2 volumes of dimethylformamide R.
Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
1 mL of 0.1 M sodium hydroxide is equivalent to 23.07 mg of C8H7ClN2O2S.