(Ph. Eur. monograph 1196)
C20H23ClN2O4 390. 2438-32-6
Action and use
Histamine H1 receptor antagonist; antihistamine.
DEFINITION
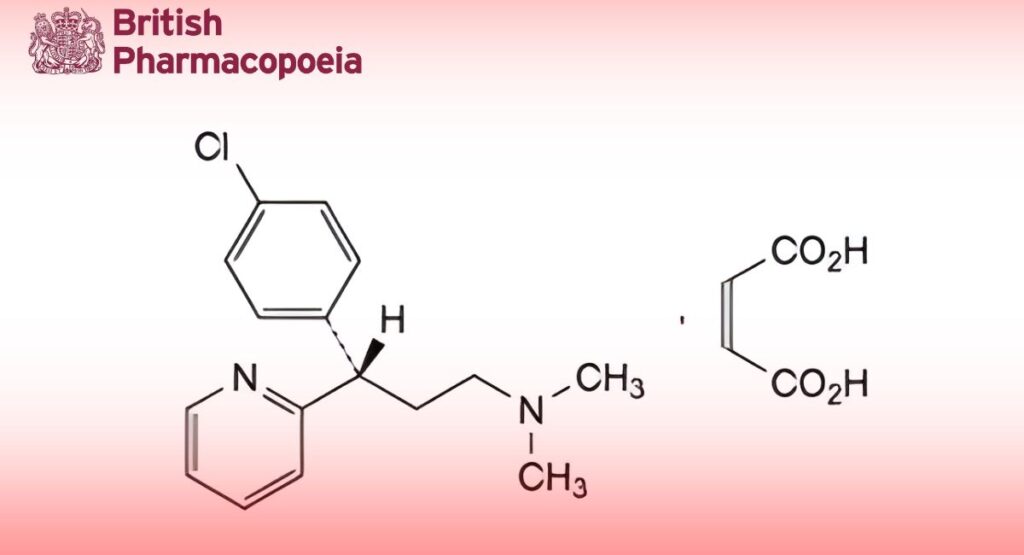
(3S)-3-(4-Chlorophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine (Z)-butenedioate.
Content
98.0 per cent to 100.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Very soluble in water, freely soluble in ethanol (96 per cent), in methanol and in methylene chloride.
IDENTIFICATION
First identification: A, C, E.
Second identification: A, B, D, E.
A. Specific optical rotation (see Tests).
B. Melting point (2.2.14): 110 °C to 115 °C.
C. Infrared absorption spectrophotometry (2.2.24).
Preparation: Discs of potassium bromide R.
Comparison: dexchlorpheniramine maleate CRS.
D. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 0.10 g of the substance to be examined in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution: Dissolve 56 mg of maleic acid R in methanol R and dilute to 10 mL with the same solvent.
Plate: TLC silica gel F254 plate R.
Mobile phase: water R, anhydrous formic acid R, methanol R, di-isopropyl ether R (3:7:20:70 V/V/V/V).
Application: 5 μL.
Development: Over a path of 12 cm.
Drying: In a current of air for a few minutes.
Detection: Examine in ultraviolet light at 254 nm.
Results: The chromatogram obtained with the test solution shows 2 clearly separated spots. The upper spot is similar in position and size to the spot in the chromatogram obtained with the reference solution.
E. To 0.15 g in a porcelain crucible add 0.5 g of anhydrous sodium carbonate R. Heat over an open flame for 10 min.
Allow to cool. Take up the residue with 10 mL of dilute nitric acid R and filter. To 1 mL of the filtrate add 1 mL of water R. The solution gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 2.0 g in water R and dilute to 20.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution BY6 (2.2.2, Method II).
pH (2.2.3)
4.5 to 5.5.
Dissolve 0.20 g in 20 mL of water R.
Specific optical rotation (2.2.7)
+ 22 to + 23 (dried substance), determined on solution S.
Related substances
Gas chromatography (2.2.28).
Test solution: Dissolve 10.0 mg of the substance to be examined in 1.0 mL of methylene chloride R.
Reference solution: Dissolve 5.0 mg of brompheniramine maleate CRS in 0.5 mL of methylene chloride R and add 0.5 mL of the test solution. Dilute 0.5 mL of this solution to 50.0 mL with methylene chloride R.
Column:
— material: glass;
— size: l = 2.3 m, Ø = 2 mm;
— stationary phase: silanised diatomaceous earth for gas chromatography R (135-175 μm) impregnated with 3 per cent m/m of phenyl(50)methyl(50)polysiloxane R.
Carrier gas: nitrogen for chromatography R.
Flow rate: 20 mL/min.
Temperature:
— column: 205 °C;
— injection port and detector: 250 °C.
Detection: Flame ionisation.
Injection: 1 μL.
Run time: 2.5 times the retention time of dexchlorpheniramine.
System suitability: Reference solution:
— resolution: minimum 1.5 between the peaks due to dexchlorpheniramine and brompheniramine.
Limits:
— impurity A: not more than 0.8 times the area of the peak due to dexchlorpheniramine in the chromatogram obtained with the reference solution (0.4 per cent);
— total: not more than twice the area of the peak due to dexchlorpheniramine in the chromatogram obtained with the reference solution (1 per cent).
Enantiomeric purity
Liquid chromatography (2.2.29).
Test solution: Dissolve 10.0 mg of the substance to be examined in 3 mL of water R. Add a few drops of concentrated ammonia R until an alkaline reaction is produced. Shake with 5 mL of methylene chloride R. Separate the layers. Evaporate the lower, methylene chloride layer to an oily residue on a water-bath. Dissolve the oily residue in 2-propanol R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 10.0 mg of dexchlorpheniramine maleate CRS in 3 mL of water R. Add a few drops of concentrated ammonia R until an alkaline reaction is produced. Shake with 5 mL of methylene chloride R. Separate the layers. Evaporate the lower, methylene chloride layer to an oily residue on a water-bath. Dissolve the oily residue in 2- propanol R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dissolve 10 mg of chlorphenamine maleate CRS in 3 mL of water R. Add a few drops of concentrated ammonia R until an alkaline reaction is produced. Shake with 5 mL of methylene chloride R. Separate the layers. Evaporate the lower, methylene chloride layer to an oily residue on a water-bath. Dissolve the oily residue in 2- propanol R and dilute to 10 mL with the same solvent.
Reference solution (c): Dilute 1.0 mL of the test solution to 50 mL with 2-propanol R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: amylose derivative of silica gel for chromatography R.
Mobile phase: diethylamine R, 2-propanol R, hexane R (3:20:980 V/V/V).
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 10 μL.
Under these conditions the peak due to the (S)-isomer appears first.
System suitability:
— resolution: minimum 1.5 between the peaks due to the (R)-enantiomer (impurity B) and the (S)-enantiomer in the chromatogram obtained with reference solution (b);
— the retention times of the principal peaks in the chromatograms obtained with the test solution and reference solution (a) are identical ((S)-enantiomer).
Limits:
— (R)-enantiomer (impurity B): not more than the area of the principal peak in the chromatogram obtained with Reference solution (c) (2 per cent);
— unspecified impurities: for each impurity, not more than 0.25 times the area of the principal peak in the
chromatogram obtained with reference solution (c) (0.5 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 65 °C for 4 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.150 g in 25 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 19.54 mg of C20H23ClN2O4.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B.
A. (3RS)-N,N-dimethyl-3-phenyl-3-(pyridin-2-yl)propan-1-amine,
B. (3R)-3-(4-chlorophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine ((R)-enantiomer).