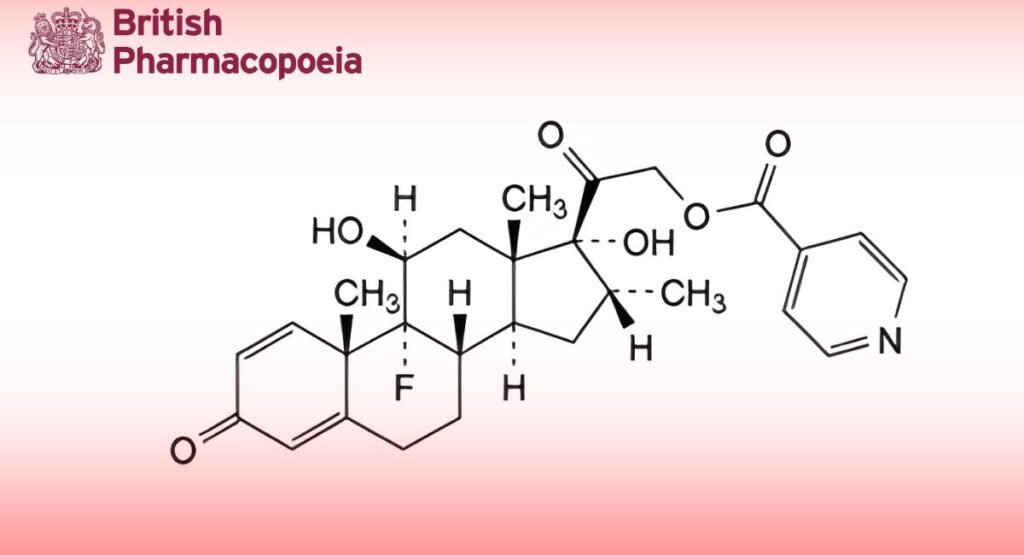
(Ph. Eur. monograph 2237)
C28H32FNO6 497.6 2265-64-7
Action and use
Glucocorticoid.
DEFINITION
9-Fluoro-11β,17-dihydroxy-16α-methyl-3,20-dioxopregna-1,4-dien-21-yl pyridine-4-carboxylate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white crystalline powder.
Solubility
Practically insoluble in water, slightly soluble in anhydrous ethanol and in acetone.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: dexamethasone isonicotinate CRS.
TESTS
Specific optical rotation (2.2.7)
+ 142 to + 146 (dried substance).
Suspend 0.200 g in 4.0 mL of ethyl acetate R and dilute to 20.0 mL with ethanol (96 per cent) R. Treat in an ultrasonic ba until a clear solution is obtained.
Related substances
Liquid chromatography (2.2.29). Prepare solutions immediately before use.
Test solution: Suspend 50.0 mg in 7 mL of acetonitrile R and dilute to 10.0 mL with water R. Treat in an ultrasonic bath until a clear solution is obtained.
Reference solution (a): Suspend 5.0 mg of dexamethasone CRS (impurity A) and 5.0 mg of dexamethasone acetate CR (impurity B) in 70 mL of acetonitrile R, add 1.0 mL of the test solution and dilute to 100.0 mL with water R. Treat in an ultrasonic bath until a clear solution is obtained.
Reference solution (b): Dilute 1.0 mL of reference solution (a) to 10.0 mL with water R.
Reference solution (c): Suspend 5 mg of dexamethasone isonicotinate for impurity C identification CRS in 0.7 mL of acetonitrile R and dilute to 1 mL with water R. Treat in an ultrasonic bath until a clear solution is obtained.
Column:
— size: l = 0.125 m, Ø = 4.0 mm,
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: water for chromatography R,
— mobile phase B: acetonitrile for chromatography R,
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 68 | 32 |
| 2 – 20 | 68 → 50 | 32 → 50 |
Flow rate: 1.2 mL/min.
Detection: Spectrophotometer at 240 nm.
Injection: 10 μL.
Identification of impurities: Use the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A and B; use the chromatogram supplied with dexamethasone isonicotinate for impurity C identification CRS an the chromatogram obtained with reference solution (c) to identify the peak due to impurity C.
Relative retention: With reference to dexamethasone isonicotinate (retention time = about 12 min): impurity A = about 0.4 impurity C = about 0.6; impurity B = about 0.8.
System suitability: Reference solution (a):
— resolution: minimum 5.0 between the peaks due to impurity B and dexamethasone isonicotinate.
Limits:
— impurity A: not more than 5 times the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.5 per cent),
— impurity B: not more than 3 times the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.3 per cent),
— impurity C: not more than 3 times the area of the peak due to dexamethasone isonicotinate in the chromatogram obtained with reference solution (b) (0.3 per cent),
— unspecified impurities: for each impurity, not more than the area of the peak due to dexamethasone isonicotinate i the chromatogram obtained with reference solution (b) (0.10 per cent),
— total: not more than 8 times the area of the peak due to dexamethasone isonicotinate in the chromatogram obtained with reference solution (b) (0.8 per cent),
— disregard limit: 0.5 times the area of the peak due to dexamethasone isonicotinate in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in vacuo at 105 °C at a pressure not exceeding 0.1 kPa for 4 h.
ASSAY
Dissolve 0.400 g in a mixture of 5 mL of anhydrous formic acid R and 50 mL of glacial acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 49.76 mg of 6C28H32FNO6.
IMPURITIES
Specified impurities A, B, C.
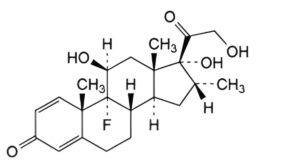
A. 9-fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione (dexamethasone),
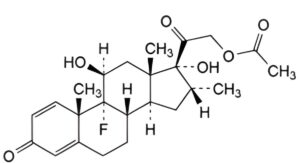
B. 9-fluoro-11β,17-dihydroxy-16α-methyl-3,20-dioxopregna-1,4-dien-21-yl acetate (dexamethasone acetate),
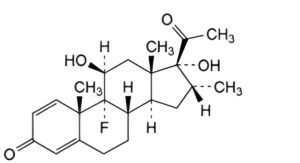
C. 9-fluoro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione (21-deoxydexamethasone).