Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
Action and use
Glucocorticoid.
DEFINITION
Dexamethasone Eye Drops, Suspension are a sterile suspension of Dexamethasone in a suitable vehicle.
The eye drops comply with the requirements stated under Eye Preparations and with the following requirements.
Content of dexamethasone, C22H29FO5
95.0 to 105.0% of the stated amount.
The eye drops should be shaken vigorously before carrying out the following tests.
IDENTIFICATION
Mix a quantity of the Eye drops containing 20 mg of Dexamethasone with 5 mL of 0.1M sodium hydroxide, add 50 mL of dichloromethane and mix with the aid of ultrasound for 20 minutes, filter the dichloromethane layer and evaporate to dryness using a rotary evaporator. Dry the residue at 105° for 2 hours. The infrared absorption spectrum of the dried residue, Appendix II A, is concordant with the reference spectrum of dexamethasone (RS 089).
TESTS
Particle size
The eye drops are a suspension and comply with the following test:
Examine using an automated light obscuration instrument such as that described in Appendix XIII A. Not more than 20 particles greater than 25 pm, not more than 2 particles greater than 50 pm and no particles greater than 90 pm.
Acidity
pH, 5.0 to 6.0, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase.
(1) Disperse a quantity of the eye drops containing 20 mg of Dexamethasone in 70 mL of mobile phase, mix with the aid of ultrasound for 10 minutes, dilute with sufficient mobile phase to produce 100 mL and filter.
(2) Dilute 3 volume of solution (1) to 100 volumes with the mobile phase.
(3) 0.02% w/v of dexamethasone impurity standard BPCRS.
(4) Dilute 1 volume of solution (1) to 100 volumes with the mobile phase, dilute 1 mL of this solution to 10 volumes with mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm X 3.9 mm) packed with octadecylsilyl silica gel for chromatography R (5 pm) (Waters Symmetry C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 50 pL of each solution.
(g) For solution (1) allow the chromatography to proceed for six times the retention time of dexamethasone.
MOBILE PHASE
27 volumes of acetonitrile and 73 volumes of a 0.3% w/v solution of orthophosphoric acid that has been previously adjusted to pH 3.0 with dilute sodium hydroxide.
SYSTEM SUITABILITY
The test is not valid unless, the chromatogram obtained with solution (3):
the resolution factor between the peaks due to impurity 3 and dexamethasone is at least 1.5;
closely resembles the chromatogram supplied with dexamethasone impurity standard BPCRS.
LIMITS
In the chromatogram obtained with solution (1):
the sum of the areas of any peaks, apart from the principal peak, is not greater than the area of the peak in the chromatogram obtained with solution (2) (3%).
Disregard any peak with an area less than the area of the peak in the chromatogram obtained with solution (4) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase.
(1) Disperse a quantity of the eye drops containing 20 mg of Dexamethasone in 70 mL of mobile phase, mix with the aid of ultrasound for 10 minutes, dilute with sufficient mobile phase to produce 100 mL and filter.
(2) 0.02% w/v of dexamethasone BPCRS.
(3) 0.01% w/v of dexamethasone impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3):
the resolution factor between the peaks due to impurity 3 and dexamethasone is at least 1.5;
closely resembles the chromatogram supplied with dexamethasone impurity standard BPCRS.
DETERMINATION OF CONTENT
Calculate the content of C22H29FO5 in the eye drops using the declared content of C22H29FO5 in dexamethasone BPCRS.
STORAGE
Dexamethasone Eye Drops, Suspension should be stored in accordance with the manufacturer’s instructions.
IMPURITIES
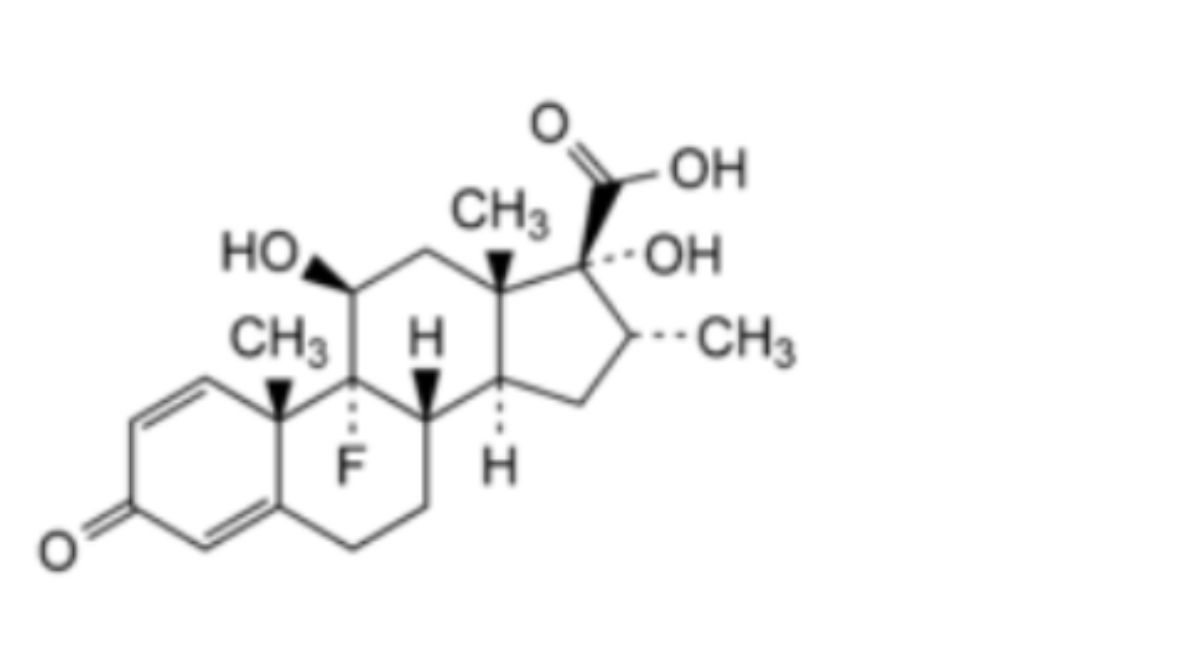
1. Dexamethasone-17β-carboxylic acid.
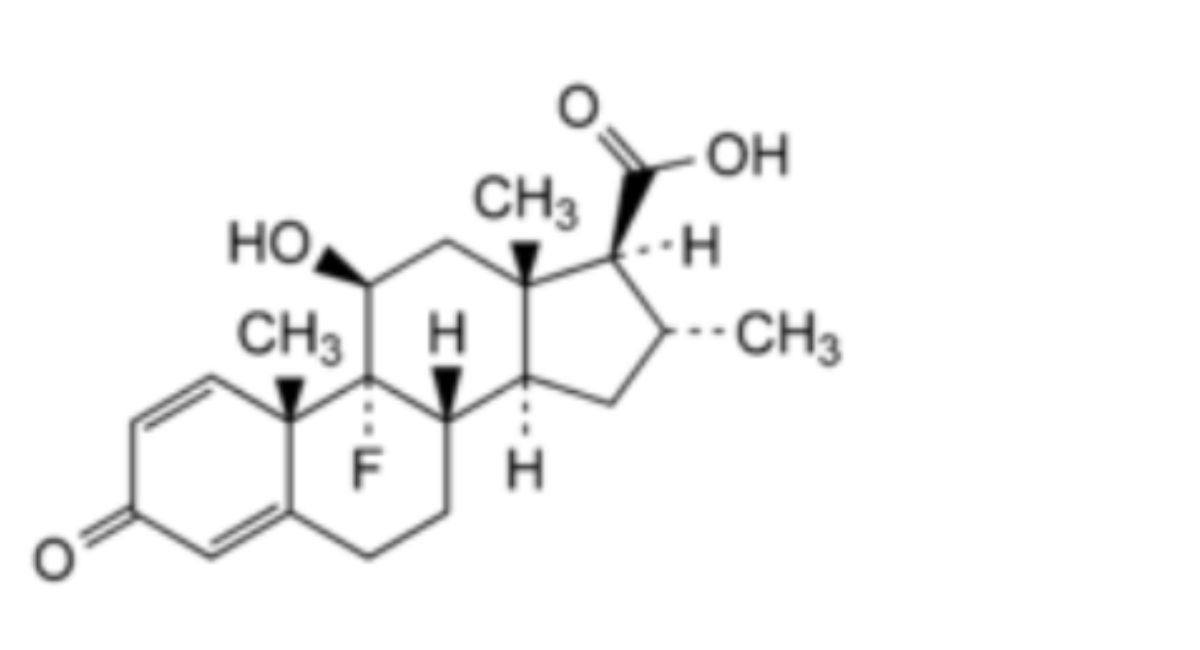
2. Dexamethasone-17α-dehydroxy-17β-carboxylic acid.
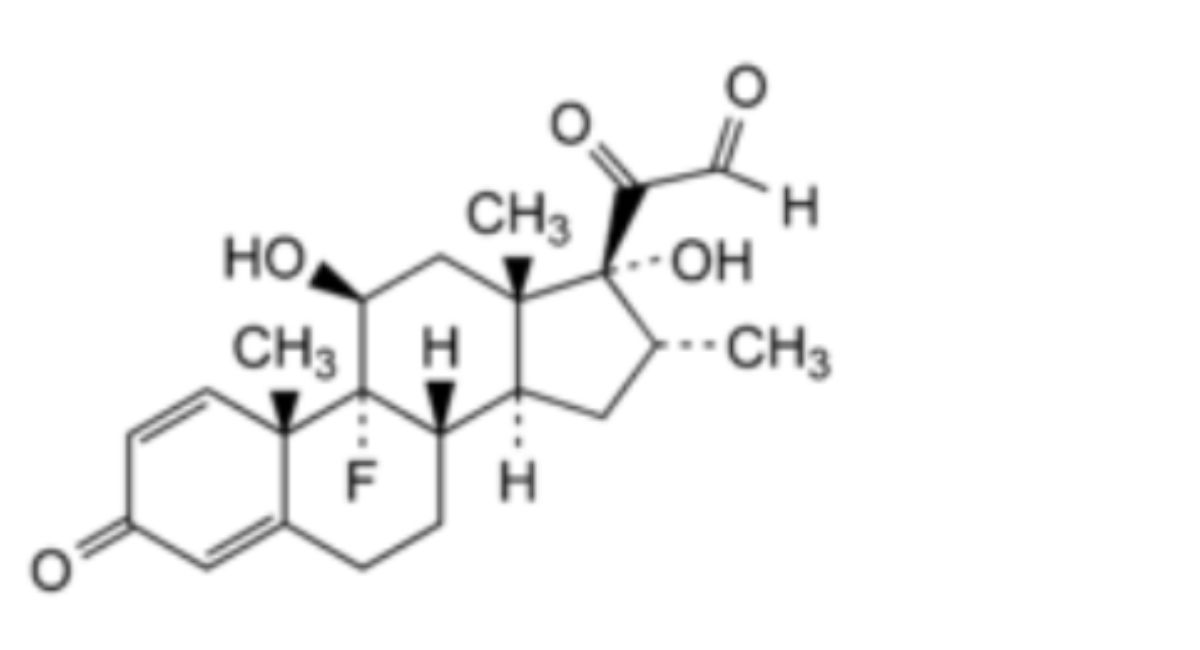
3. Dexamethasone-21-aldehyde.
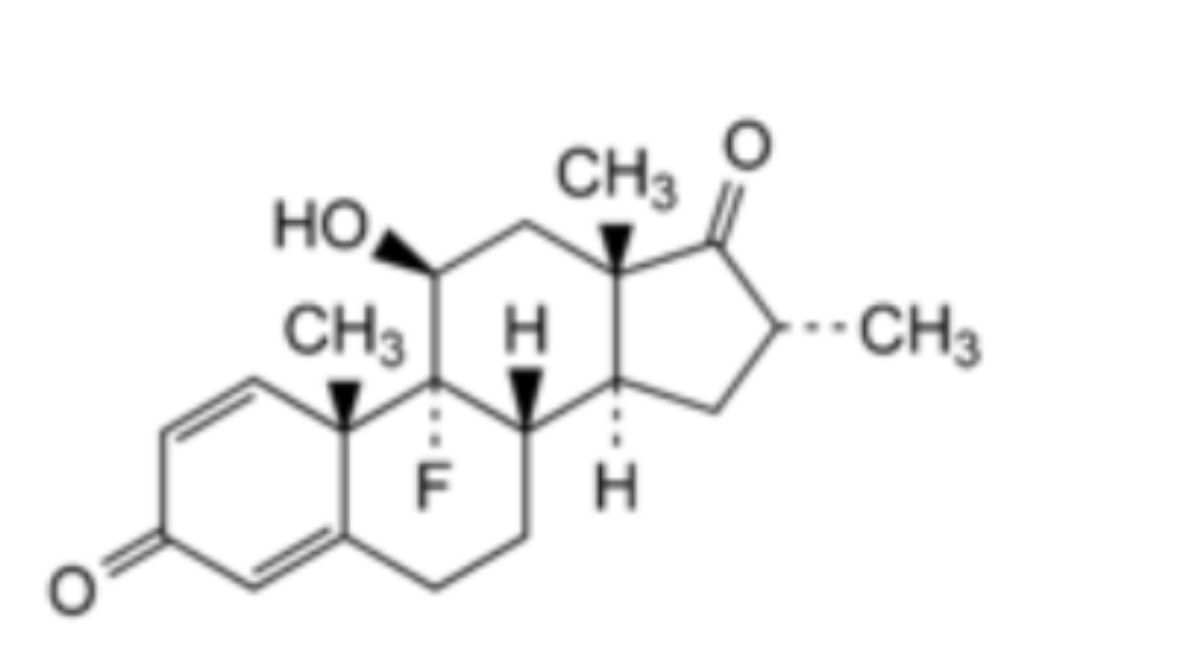
4. Dexamethasone-17-ketone.
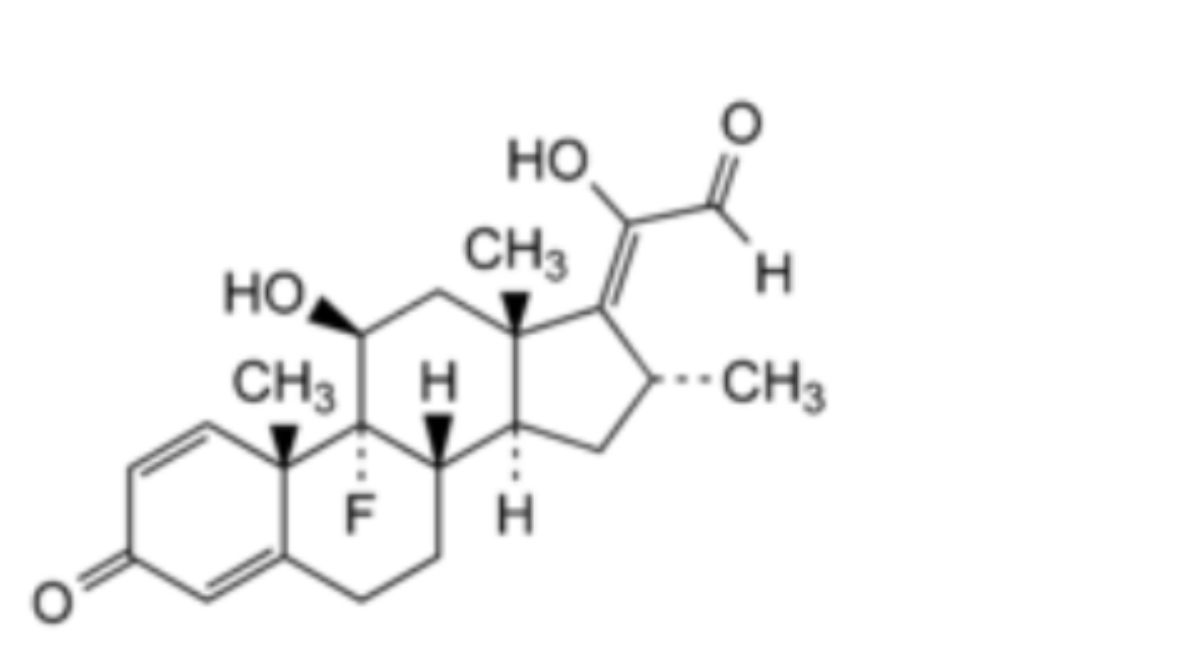
5. Dexamethasone-17(20)-enol-21-aldehyde.






