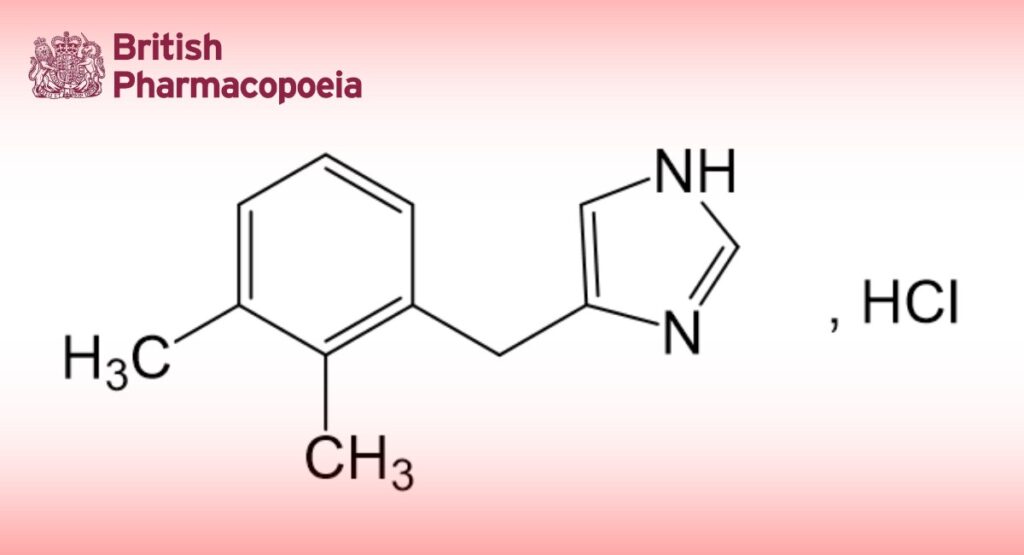
Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Alpha2-adrenoceptor agonist.
DEFINITION
4-[(2,3-Dimethylphenyl)methyl]-1H-imidazole hydrochloride.
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, hygroscopic, crystalline powder.
Solubility
Soluble in water, freely soluble in ethanol (96 per cent), very slightly soluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison detomidine hydrochloride CRS.
If the spectra obtained show differences, dry the substance to be examined and the reference substance separately in an oven at 105 °C and record new spectra.
B. It gives reaction (a) of chlorides (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II). Dissolve 0.25 g in water R and dilute to 25 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 25 mg of the substance to be examined in 20 mL of the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (a) Dilute 0.20 mL of the test solution to 100.0 mL with the mobile phase.
Reference solution (b) Dissolve 1 mg of detomidine impurity B CRS in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 10.0 mL with reference solution (a).
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography R (5 µm).
Mobile phase acetonitrile R1, 2.64 g/L solution of ammonium phosphate R (35:65 V/V). Flow rate 1 mL/min.
Detection Spectrophotometer at 220 nm.
Injection 20 µL.
Run time 4 times the retention time of detomidine.
Identification of impurities Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity B.
Relative retention With reference to detomidine (retention time = about 6 min): impurity B = about 2.1.
System suitability Reference solution (b):
— resolution: minimum 5.0 between the peaks due to detomidine and impurity B.
Calculation of percentage contents:
— for each impurity, use the concentration of detomidine hydrochloride in reference solution (a).
Limits:
— unspecified impurities: for each impurity, maximum 0.20 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.10 per cent.
Water (2.5.12)
Maximum 2.0 per cent, determined on 0.250 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.170 g in 50 mL of ethanol (96 per cent) R. Add 5.0 mL of 0.01 M hydrochloric acid. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 22.27 mg of C12H15ClN2.
STORAGE
In an airtight container.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C.
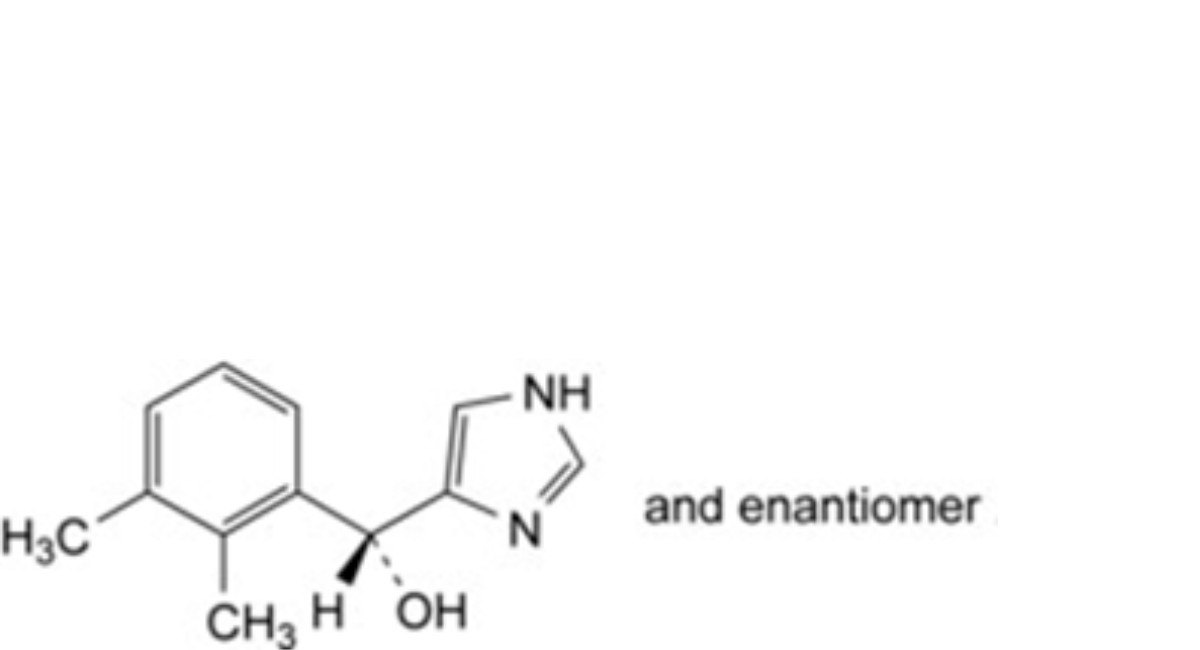
A. (RS)-(2,3-dimethylphenyl)(1H-imidazol-4-yl)methanol,
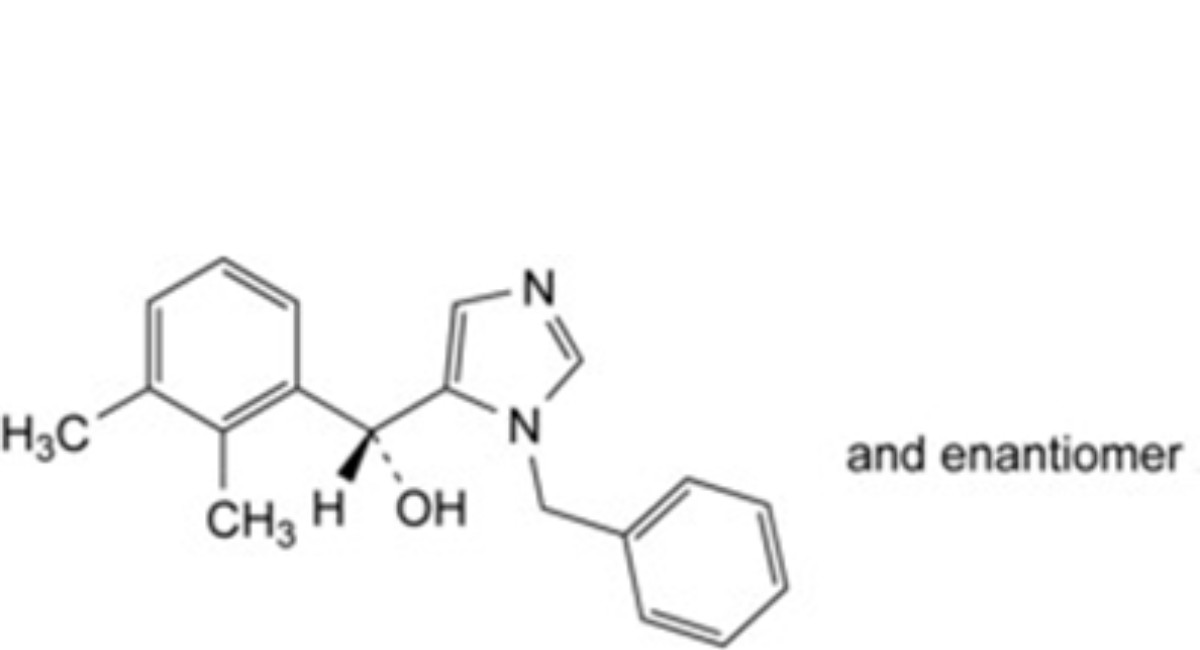
B. (RS)-(1-benzyl-1H-imidazol-5-yl)(2,3-dimethylphenyl)methanol,
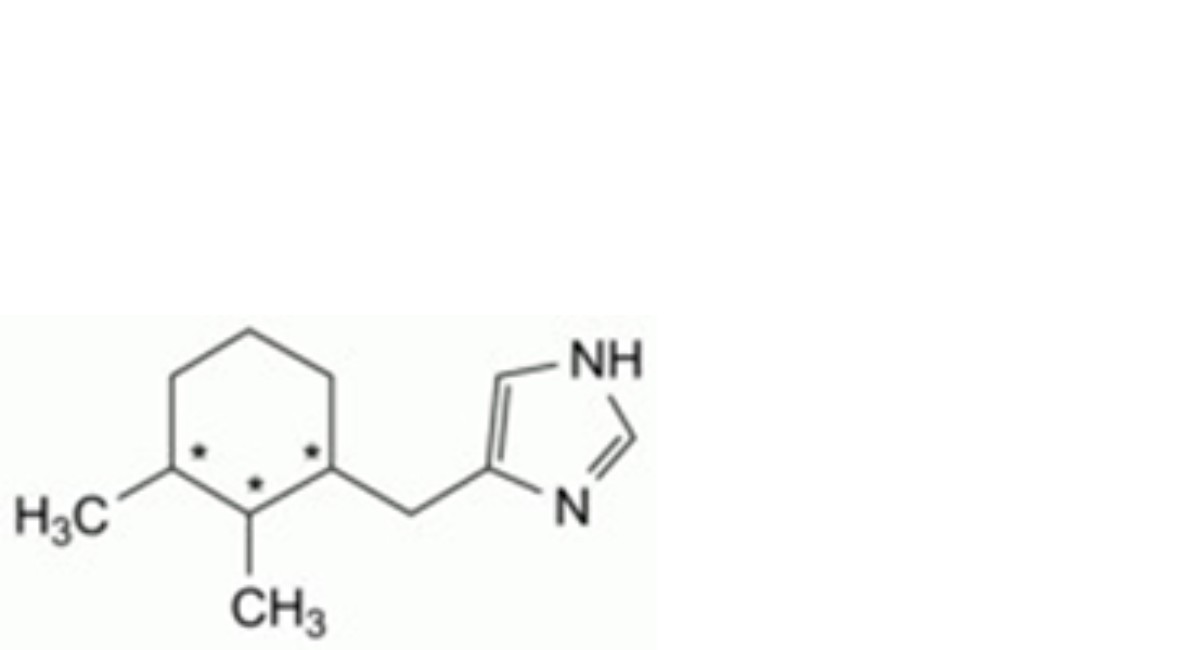
C. 4-[(2,3-dimethylcyclohexyl)methyl]-1H-imidazole.