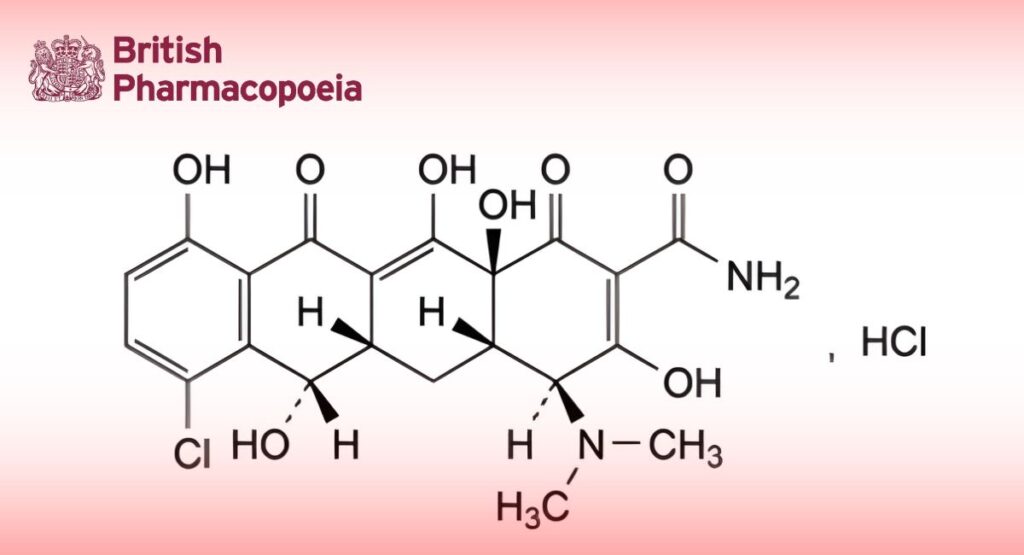
(Ph. Eur. monograph 0176)
C21H22Cl2N2O8 501.3 64-73-3
Action and use
Tetracycline antibacterial.
Preparation
Demeclocycline Capsules
DEFINITION
(4S,4aS,5aS,6S,12aS)-7-Chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide hydrochloride.
Substance produced by certain strains of Streptomyces aureofaciens.
Content
89.5 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
Yellow powder.
Solubility
Soluble or sparingly soluble in water, slightly soluble in ethanol (96 per cent), very slightly soluble in acetone. It dissolves in solutions of alkali hydroxides and carbonates.
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 5 mg of the substance to be examined in methanol R and dilute to 10 mL with the same solvent.
Reference solution (a): Dissolve 5 mg of demeclocycline hydrochloride CRS in methanol R and dilute to 10 mL with the same solvent.
Reference solution (b): Dissolve 5 mg of demeclocycline hydrochloride CRS, 5 mg of chlortetracycline hydrochloride R and 5 mg of tetracycline hydrochloride R in methanol R and dilute to 10 mL with the same solvent.
Plate: TLC octadecylsilyl silica gel F254 plate R.
Mobile phase: Mix 20 volumes of acetonitrile R, 20 volumes of methanol R and 60 volumes of a 63 g/L solution of oxalic acid R previously adjusted to pH 2 with concentrated ammonia R.
Application: 1 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
System suitability: The chromatogram obtained with reference solution (b) shows 3 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
B. To about 2 mg add 5 mL of sulfuric acid R. A violet colour develops. Add the solution to 2.5 mL of water R. The colour becomes yellow.
C. It gives reaction (a) of chlorides (2.3.1).
TESTS
pH (2.2.3)
2.0 to 3.0.
Dissolve 0.1 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Buffer 1 22.2 g/L solution of sodium edetate R adjusted to pH 7.5 with a 40 g/L solution of sodium hydroxide R.
Buffer 2 17.0 g/L solution of tetrapropylammonium hydrogen sulfate R adjusted to pH 7.5 with a 40 g/L solution of sodium hydroxide R.
Test solution: Dissolve 25.0 mg of the substance to be examined in a 1.0 g/L solution of hydrochloric acid R and dilute to 50.0 mL with the same solution.
Reference solution (a): Dissolve 25.0 mg of demeclocycline hydrochloride CRS in a 1.0 g/L solution of hydrochloric acid R and dilute to 50.0 mL with the same solution.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with a 1.0 g/L solution of hydrochloric acid R.
Reference solution (c): Dissolve 5 mg of demeclocycline for system suitability CRS (containing impurities A, B, C, E and G) in a 1.0 g/L solution of hydrochloric acid R and dilute to 10 mL with the same solution.
Column:
— size: l = 0.075 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography with embedded polar groups R (3.5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: acetonitrile R, water for chromatography R, buffer 1, buffer 2 (2:28:35:35 V/V/V/V);
— mobile phase B: acetonitrile R, buffer 1, buffer 2 (30:35:35 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 5 | 83 | 17 |
| 5 – 15 | 83 → 30 | 17 → 70 |
| 15 – 25 | 30 | 70 |
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 280 nm.
Injection: 10 μL of the test solution and reference solutions (b) and (c).
Identification of impurities: Use the chromatogram supplied with demeclocycline for system suitability CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A, B, C, E and G.
Relative retention: With reference to demeclocycline (retention time = about 14 min): impurity C = about 0.3; impurity B = about 0.7; impurity A = about 0.8; impurity E = about 1.2; impurity G = about 1.6.
System suitability: Reference solution (c):
— resolution: minimum 2.5 between the peaks due to impurities A and B.
Calculation of percentage contents:
— for each impurity, use the concentration of demeclocycline hydrochloride in reference solution (b).
Limits:
— impurities A, B: for each impurity, maximum 5.0 per cent;
— impurities C, G: for each impurity, maximum 0.3 per cent;
— impurity E: maximum 0.2 per cent;
— any other impurity: for each impurity, maximum 0.15 per cent;
— total: maximum 10.0 per cent;
— reporting threshold: 0.05 per cent.
Water (2.5.12)
Maximum 3.0 per cent, determined on 1.000 g.
Sulfated ash (2.4.14)
Maximum 0.5 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: 10 μL of the test solution and reference solution (a).
Calculate the percentage content of C21H22Cl2N2O8 using the chromatogram obtained with reference solution (a) and taking into account the assigned content of demeclocycline hydrochloride CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, E, G.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) D, F.
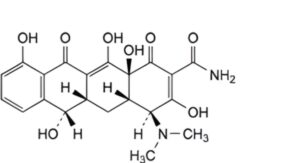
A. (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (demethyltetracycline),
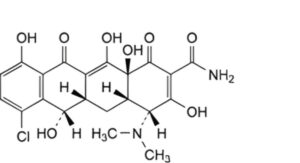
B. (4R,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epidemeclocycline),
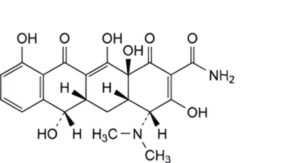
C. (4R,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epidemethyltetracycline),
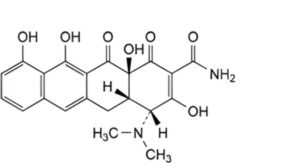
D. (4R,4aS,12aS)-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-1,12-dioxo-1,4,4a,5,12,12a-hexahydrotetracene-2-carboxamide (4-epianhydrodemethyltetracycline),
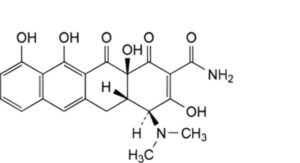
E. (4S,4aS,12aS)-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-1,12-dioxo-1,4,4a,5,12,12a-hexahydrotetracene-2-carboxamide (anhydrodemethyltetracycline),
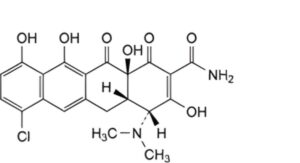
F. (4R,4aS,12aS)-7-chloro-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-1,12-dioxo-1,4,4a,5,12,12a-hexahydrotetracene-2-carboxamide (4-epianhydrodemeclocycline),
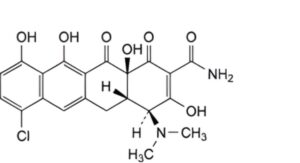
G. (4S,4aS,12aS)-7-chloro-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-1,12-dioxo-1,4,4a,5,12,12a-hexahydrotetracene-2-carboxamide (anhydrodemeclocycline).