Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Insecticide (veterinary).
Preparation
Deltamethrin Pour-on
DEFINITION
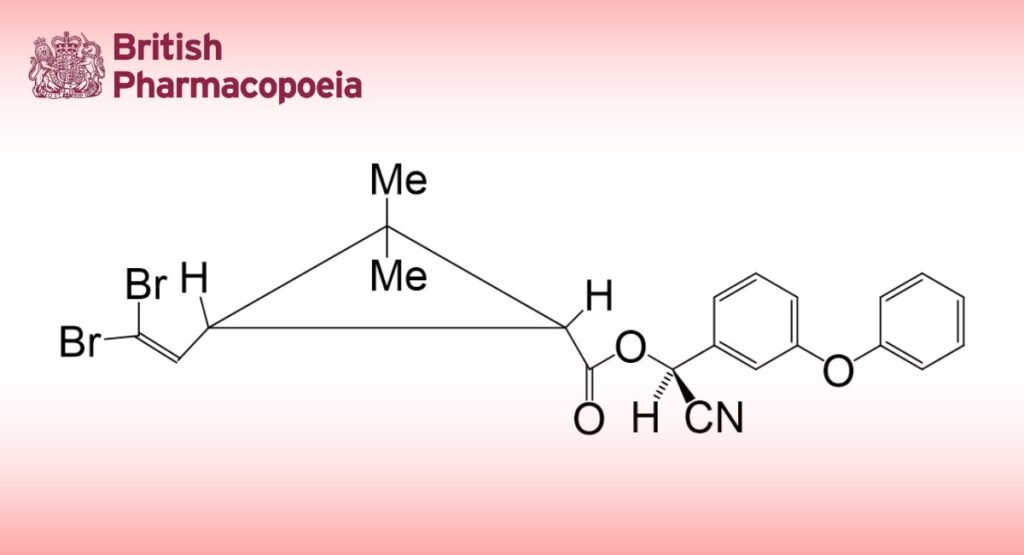
Deltamethrin is (S)-α-cyano-3-phenoxybenzyl-(1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylate. It contains not less than 97.0% and not more than 101.0% of C22H19Br2NO3.
CHARACTERISTICS
A white to buff-coloured, crystalline powder.
Insoluble in water; soluble in ethanol (96%) and in acetone.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of deltamethrin (RSV 47).
B. In the test for Related substances, the principal spot in the chromatogram obtained with solution (2) corresponds to that in the chromatogram obtained with solution (5).
TESTS
Specific optical rotation
In a 4% w/v solution in toluene, +55.5 to +61.5, Appendix V F.
Becisthemic acid chloride
Not more than 0.2% when determined by the following method. Dissolve 2 g in 100 ml of methanol with moderate heating, if necessary, and cool. Titrate with 0.02M potassium hydroxide VS using a solution containing 0.8% w/v of dimethyl yellow and 0.08% w/v of methylene blue in methanol as indicator to a green end point. Each ml of 0.02M potassium hydroxide VS is equivalent to 6.329 mg of becisthemic acid chloride, C8H9Br2ClO.
Becisthemic acid and becisthemic anhydride
Not more than 1% in total when determined by the following methods.
Becisthemic acid
Dissolve 2 g in 100 ml of ethanol (96%) with moderate heating. Cool in an ice-bath and immediately titrate with 0.02M sodium hydroxide VS using a 1% w/v solution of 1-naphtholbenzein in ethanol (96%) solution as indicator to a green end point. Correct the volume of titrant for any contribution due to the becisthemic acid chloride content using the following expression:
V × P2/P1
where V = titration volume obtained in the becisthemic acid chloride test,
P1 = weight of sample used in the becisthemic chloride test,
P2 = weight of sample used in this test.
Each ml of 0.02M sodium hydroxide VS is equivalent to 5.959 mg of becisthemic acid, C8H10Br2O2.
Becisthemic anhydride
To 1.0 g add 10 ml of 0.01M aniline in cyclohexane and 10 ml of glacial acetic acid. Stopper the flask and allow to stand at room temperature for 1 hour. Titrate with 0.01M perchloric acid VS using crystal violet solution as indicator. Repeat the procedure omitting the substance being examined. Correct the volume of titrant for any contribution due to twice the becisthemic acid chloride content calculated using the above formula. Each ml of 0.01M perchloric acid VS is equivalent to 5.779 mg of becisthemic anhydride, C16H18Br4O3.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in toluene.
(1) 2.0% w/v of the substance being examined.
(2) 0.5% w/v of the substance being examined.
(3) 0.020% w/v of the substance being examined.
(4) 0.010% w/v of the substance being examined.
(5) 0.5% w/v of deltamethrin BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a silica gel F254 precoated plate (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 10 µl of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
20 volumes of di-isopropyl ether and 80 volumes of hexane.
LIMITS
Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (3) (1%) and not more than two such spots are more intense than the spot in the chromatogram obtained with solution (4) (0.5%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase.
(1) 0.1% w/v of the substance being examined.
(2) 0.1% w/v of deltamethrin BPCRS.
(3) 0.1% w/v of deltamethrin impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with silica gel for chromatography (5 µm) (Zorbax Sil is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.3 ml per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 278 nm.
(f) Inject 20 µl of each solution.
MOBILE PHASE
0.04 volume of propan-2-ol, 2 volumes of acetonitrile, 10 volumes of dichloromethane and 100 volumes of hexane.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), a peak due to (R)-deltamethrin appears immediately before the principal peak, as indicated in the reference chromatogram supplied with deltamethrin impurity standard BPCRS.
DETERMINATION OF CONTENT
Calculate the content of C22H19Br2NO3 using the declared content of C22H19Br2NO3 in deltamethrin BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include:
— Becisthemic acid,
— Becisthemic anhydride,
— Becisthemic acid chloride.