Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Antiprotozoal (veterinary). Preparation Decoquinate Premix
DEFINITION
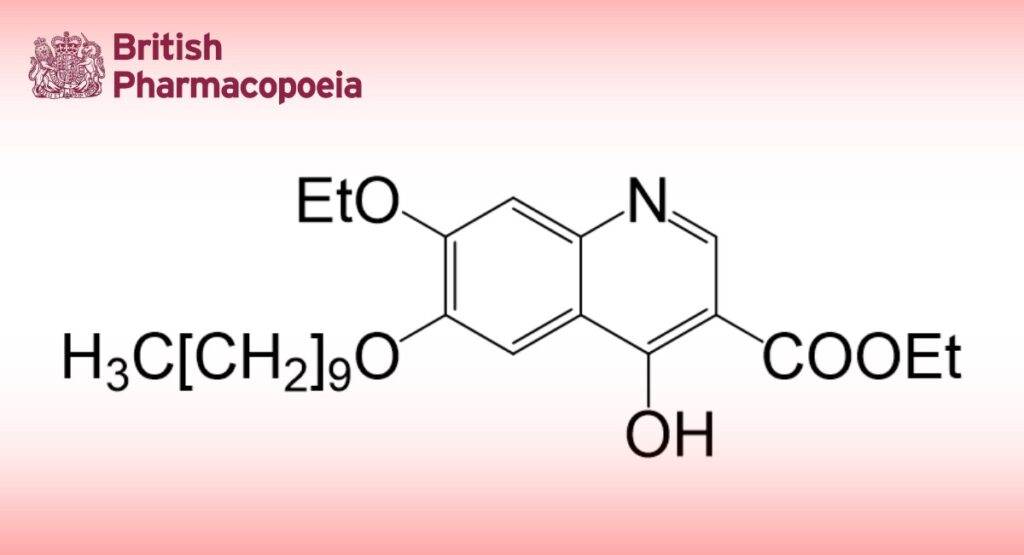
Decoquinate is ethyl 6-decyloxy-7-ethoxy-4-hydroxyquinoline-3-carboxylate. It contains not less than 99.0% and not more than 101.0% of C24H35NO5, calculated with reference to the dried substance.
CHARACTERISTICS
A cream to buff-coloured, microcrystalline powder; odourless or almost odourless.
Insoluble in water; very slightly soluble in chloroform and in ether; practically insoluble in ethanol (96%).
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of decoquinate (RSV 14).
B. The light absorption, Appendix II B, in the range 230 to 350 nm of the solution used in the test for Light absorption exhibits a well-defined maximum only at 265 nm.
TESTS
Light absorption
Dissolve 40 mg in 10 mL of hot chloroform and, keeping the solution warm, dilute slowly with 70 mL of absolute ethanol. Cool and dilute to 100 mL with absolute ethanol. Immediately dilute 10 mL to 100 mL with absolute ethanol. To 10 mL of the solution add 10 mL of 0.1M hydrochloric acid and dilute to 100 mL with absolute ethanol. The absorbance of the resulting solution at the maximum at 265 nm is 0.38 to 0.42, calculated with reference to the dried substance, Appendix II B.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) 1.0% w/v of the substance being examined in chloroform, prepared with the aid of heat.
(2) 0.0050% w/v of diethyl 4-decyloxy-3-ethoxyanilinomethylene malonate BPCRS in chloroform.
(3) 0.010% w/v of the substance being examined in chloroform.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 10 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
5 volumes of anhydrous formic acid, 10 volumes of absolute ethanol and 85 volumes of chloroform.
LIMITS
Any secondary spot corresponding to diethyl 4-decyloxy-3-ethoxyanilinomethylene malonate in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (0.5%) and any other secondary spot is not more intense than the spot in the chromatogram obtained with solution (3) (1%).
Loss on drying
When dried to constant weight at 105°, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 1 g in a mixture of 50 mL of chloroform and 50 mL of anhydrous acetic acid and carry out Method I for non- aqueous titration, Appendix VIII A, using crystal violet solution as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 41.76 mg of C24H35NO5.