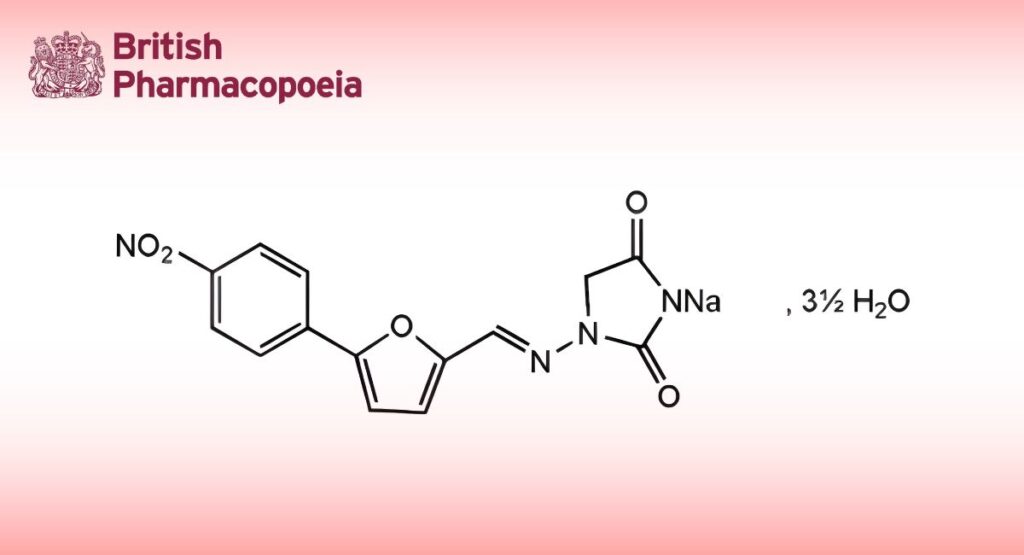
C14H9N4NaO5,31⁄2H2O 399.3 24868-20-0
Action and use
Skeletal muscle relaxant.
Preparation
Dantrolene Oral Suspension
DEFINITION
Dantrolene Sodium is 1-(5-p-nitrophenylfurfurylideneamino)hydantoin sodium. It contains not less than 98.0% and not more than 102.0% of C14H9N4NaO5, calculated with reference to the anhydrous substance.
CHARACTERISTICS
A yellowish-orange to orange crystalline powder.
Very slightly soluble in water; slightly soluble in ethanol (96%); sparingly soluble in methanol; practically insoluble in acetone.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of dantrolene sodium (RS422).
B. In the Assay, the chromatogram obtained with solution (1) shows a peak with the same retention time as the principal peak in the chromatogram obtained with solution (2).
C. To 0.1 g of the substance being examined add 20 mL of water and 2 drops of acetic acid, shake well and filter. The filtrate yields the reactions characteristic of sodium salts, Appendix VI.
TESTS
Alkalinity
Shake 0.7 g in 10 mL of water for 5 minutes and centrifuge. To 5 mL of the supernatant add 45 mL of water and 3 drops of phenolphthalein solution R1 and 0.1 mL of 0.1M hydrochloric acid VS. A red colour is not produced.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dissolve 50 mg of the substance being examined in 20 mL of tetrahydrofuran and 2 mL of glacial acetic acid and dilute with sufficient absolute ethanol to produce 100 mL.
(2) Dilute 1 mL of solution (1) to 100 mL with absolute ethanol.
(3) Dissolve 5 mg of dantrolene sodium BPCRS and 0.1 g of theophylline BPCRS in 20 mL of tetrahydrofuran and 2 mL of glacial acetic acid and dilute with sufficient absolute ethanol to produce 100 mL. Further dilute 10 mL of this solution to 100 mL with absolute ethanol.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with silica gel for chromatography (5 μm) (Zorbax Sil is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Adjust the flow rate of the mobile phase so that the retention time of the peak corresponding to Dantrolene Sodium is about 8 minutes.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 300 nm.
(f) Inject 10 μL of each solution.
(g) For solution (1) allow the chromatography to proceed for at least twice the retention time of the principal peak.
MOBILE PHASE
9 volumes of absolute ethanol, 10 volumes of glacial acetic acid and 90 volumes of hexane.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks
corresponding to theophylline and dantrolene is at least 6.
LIMITS
In the chromatogram obtained with solution (1):
the total area of all the secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Water
14.5 to 17.0% w/w, Appendix IX C. Use 0.2 g
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dissolve 60 mg of the substance being examined in 50 mL of dimethylformamide and dilute 1 volume of the resulting solution to 100 volumes with the mobile phase.
(2) Dilute 1 volume of a 0.12% w/v solution of dantrolene sodium BPCRS in dimethylformamide to 100 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with spherical particles of silica, 5 μm in diameter, the surface of which has been modified with chemically-bonded nitrile groups (Spherisorb CN is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 262 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
15 volumes of acetonitrile and 85 volumes of a phosphate buffer pH 6.8 prepared by dissolving 11.88 g of disodium hydrogen orthophosphate and 9.08 g of potassium dihydrogen orthophosphate in 1000 mL of water.
DETERMINATION OF CONTENT
Calculate the content of C14H9N4NaO5 in the substance being examined using the declared content of C14H9N4NaO5 in dantrolene sodium BPCRS.