(Ph. Eur. monograph 1195)
Action and use
Low molecular weight heparin.
Preparation
Dalteparin Sodium Injection
DEFINITION
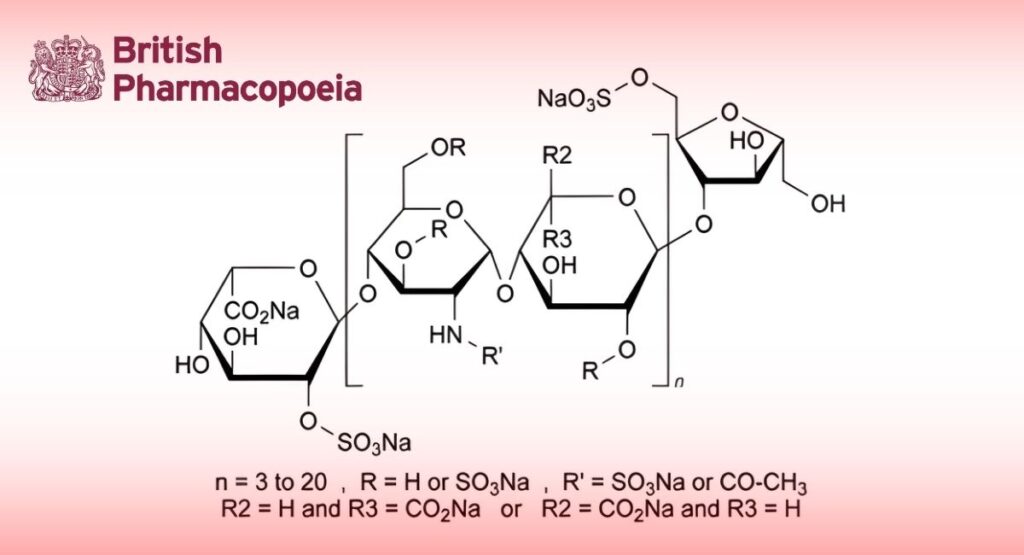
Dalteparin sodium is the sodium salt of a low-molecular-mass heparin that is obtained by nitrous acid depolymerisation of heparin from porcine intestinal mucosa. The majority of the components have a 2-O-sulfo-α-L-idopyranosuronic acid structure at the non-reducing end and a 6-O-sulfo-2,5-anhydro-D-mannitol structure at the reducing end of their chain.
Dalteparin sodium complies with the monograph Low-molecular-mass heparins (0828) with the modifications and additional requirements below.
The mass-average relative molecular mass ranges between 5600 and 6400, with a characteristic value of about 6000.
The degree of sulfatation is 2.0 to 2.5 per disaccharide unit.
The potency is not less than 110 IU and not more than 210 IU of anti-factor Xa activity per milligram, calculated with reference to the dried substance. The anti-factor IIa activity is not less than 35 IU/mg and not more than 100 IU/mg, calculated with reference to the dried substance. The ratio of anti-factor Xa activity to anti-factor IIa activity is between 1.9
and 3.2.
PRODUCTION
Dalteparin sodium is produced by a validated manufacturing and purification procedure under conditions designed to minimise the presence of N-NO groups.
The manufacturing procedure must have been shown to reduce any contamination by N-NO groups to approved limits using an appropriate, validated quantification method.
IDENTIFICATION
Carry out identification test A as described in the monograph Low-molecular-mass heparins (0828) using dalteparin sodium CRS.
Carry out identification test C as described in the monograph Low-molecular-mass heparins (0828). The following requirements apply.
The mass-average relative molecular mass ranges between 5600 and 6400. The mass percentage of chains lower than 3000 is not more than 13.0 per cent. The mass percentage of chains higher than 8000 ranges between 15.0 per cent and 25.0 per cent.
TESTS
Appearance of solution
Dissolve 1 g in 10 mL of water R. The solution is clear (2.2.1) and not more intensely coloured than intensity 5 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
Nitrite
Not more than 5 ppm. Examine by liquid chromatography (2.2.29). Rinse all volumetric flasks at least three times with water R before the preparation of the solutions.
Test solution: Dissolve 80.0 mg of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Allow to stand for at least 30 min.
Reference solution (a): Dissolve 60.0 mg of sodium nitrite R in water R and dilute to 1000.0 mL with the same solvent.
For the preparation of reference solution (b), use a pipette previously rinsed with reference solution (a).
Reference solution (b): Dilute 1.00 mL of reference solution (a) to 50.0 mL with water R.
Before preparing reference solutions (c), (d) and (e), rinse all pipettes with reference solution (b).
Reference solution (c): Dilute 1.00 mL of reference solution (b) to 100.0 mL with water R (corresponding to 1 ppm of nitrite in the test sample).
Reference solution (d): Dilute 3.00 mL of reference solution (b) to 100.0 mL with water R (corresponding to 3 ppm of nitrite in the test sample).
Reference solution (e): Dilute 5.00 mL of reference solution (b) to 100.0 mL with water R (corresponding to 5 ppm of nitrite in the test sample).
The chromatographic procedure may be carried out using:
— a column 0.125 m long and 4.3 mm in internal diameter packed with a strong anion-exchange resin;
— as mobile phase at a flow rate of 1.0 mL/min a solution consisting of 13.61 g of sodium acetate R dissolved in water R, adjusted to pH 4.3 with phosphoric acid R and diluted to 1000 mL with water R;
— as detector an appropriate electrochemical device with the following characteristics and settings: a suitable working electrode, a detector potential of + 1.00 V versus Ag/AgCl reference electrode and a detector sensitivity of 0.1 μA full scale.
Inject 100 μL of reference solution (d). When the chromatograms are recorded in the prescribed conditions, the retention time for nitrite is 3.3 to 4.0 min. The test is not valid unless:
— the number of theoretical plates calculated for the nitrite peak is at least 7000 per metre per column (dalteparin sodium will block the binding sites of the stationary phase, which will cause shorter retention times and lower separation efficiency for the analyte; the initial performance of the column may be partially restored using a 58 g/L solution of sodium chloride R at a flow rate of 1.0 mL/min for 1 h; after regeneration the column is rinsed with 200 mL to 400 mL of water R);
— the symmetry factor for the nitrite peak is less than 3;
— the relative standard deviation of the peak area for nitrite obtained from 6 injections is less than 3.0 per cent.
Inject 100 μL each of reference solutions (c) and (e). The test is not valid unless:
— the correlation factor for a linear relationship between concentration and response for reference solutions (c), (d) and (e) is at least 0.995;
— the signal-to-noise ratio for reference solution (c) is not less than 5 (if the noise level is too high, electrode recalibration is recommended);
— a blank injection of water R does not give rise to spurious peaks.
Inject 100 μL of the test solution. Calculate the content of nitrite from the peak areas in the chromatogram obtained with reference solutions (c), (d) and (e).
Boron
Not more than 1 ppm, determined by inductively coupled plasma atomic emission spectroscopy.
Boron is determined by measurement of the emission from an inductively coupled plasma (ICP) at a wavelength specific to boron. The emission line at 249.733 nm is used. Use an appropriate apparatus, whose settings have been optimised as directed by the manufacturer.
Test solution: Dissolve 0.2500 g of the substance to be examined in about 2 mL of water for chromatography R, add 100 μL of nitric acid R and dilute to 10.00 mL with the same solvent.
Reference solution (a): Prepare a 1 per cent V/V solution of nitric acid R in water for chromatography R (blank).
Reference solution (b): Prepare a 11.4 μg/mL solution of boric acid R in a 1 per cent V/V solution of nitric acid R in water for chromatography R (STDcal).
Reference solution (c): Dissolve 0.2500 g of a reference dalteparin sodium with no detectable boron in about 2 mL of water for chromatography R, add 100 μL of nitric acid R and dilute to 10.00 mL with the same solvent (STD0).
Reference solution (d): Dissolve 0.2500 g of a reference dalteparin sodium with no boron detected in about 2 mL of a 1 per cent V/V solution of nitric acid R in water for chromatography R, add 10 μL of a 5.7 mg/mL solution of boric acid R and dilute to 10.00 mL with the same solvent (STD1). This solution contains 1 μg/mL of boron.
Calculate the content of boron in the substance to be examined, using the following correction factor:
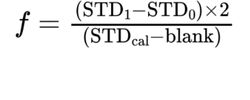
Loss on drying (2.2.32)
Not more than 5.0 per cent, determined on 1.000 g by drying in an oven at 60 °C over diphosphorus pentoxide R at a pressure not exceeding 670 Pa for 3 h.