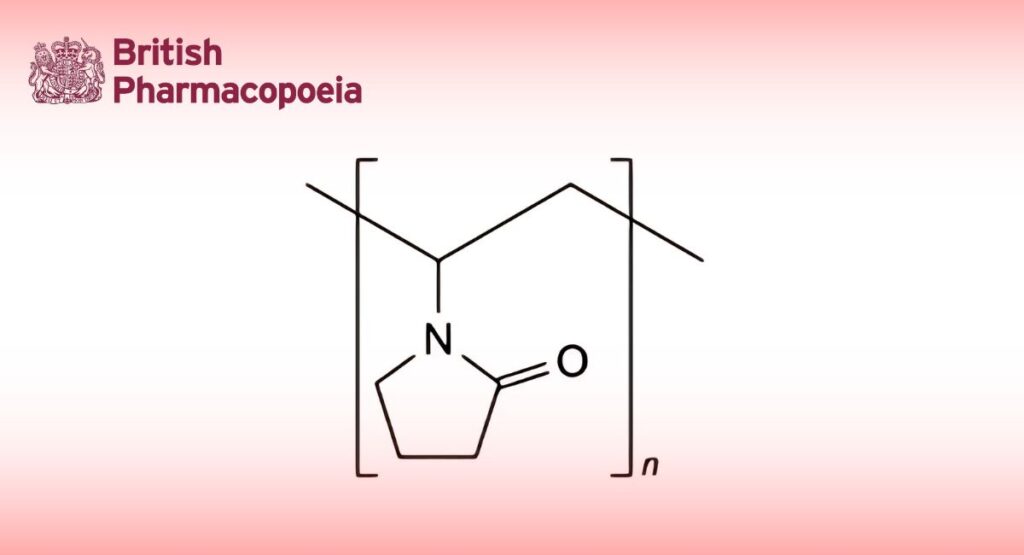
(Ph. Eur. monograph 0892)
(C6H9NO)n Mr (111.1)n 9003-39-8
Action and use
Excipient in pharmaceutical products.
DEFINITION
Cross-linked homopolymer of 1-ethenylpyrrolidin-2-one.
Content
11.0 per cent to 12.8 per cent of N (Ar 14.01) (dried substance).
2 types of crospovidone are available, depending on the particle size: type A and type B.
♦ CHARACTERS
Appearance
Hygroscopic, white or yellowish-white powder or flakes.
Solubility
Practically insoluble in water, in ethanol 96 per cent and in methylene chloride.♦
IDENTIFICATION
♦ A. Infrared absorption spectrophotometry (2.2.24).
Comparison crospovidone CRS.♦
B. Suspend 1 g in 10 mL of water R, add 0.1 mL of 0.05 M iodine and shake for 30 s. Add 1 mL of starch solution R and shake. No blue colour develops within 30 s.
C. To 10 mL of water R, add 0.1 g and shake. A suspension is formed and no clear solution is obtained within 15 min.
D. The analytical sieves must be clean and dry. For this purpose the sieves are washed in hot water and allowed to dry overnight in a drying cabinet at 105 °C.
Place 20 g (dried substance) in a 1000 mL conical flask, add 500 mL of water R and shake the suspension for 30 min. Pour the suspension through a 63 μm analytical sieve, previously tared, and rinse the sieve with water R until the filtrate is clear. Dry the sieve and sample residue at 105 °C for 5 h in a drying cabinet without circulating air. Cool in a desiccator for 30 min and weigh.
Calculate the percentage sieving residue (fraction of sample particles having a diameter of more than 63 μm), using the following expression:
m1-m3/m2x100
m1 = mass of the sieve and sample residue, after drying for 5 h, in grams;
m2 = initial mass of the sample, in grams;
m3 = mass of the sieve, in grams.
If the sieving residue fraction is more than 15 per cent, the substance is classified as type A; if the sieving residue fraction is less than or equal to 15 per cent, the substance is classified as type B.
TESTS
Peroxides
Type A: maximum 400 ppm expressed as H2O2; type B: maximum 1000 ppm expressed as H2O2.
Suspend 2.0 g in 50 mL of water R. To 25 mL of this suspension add 2 mL of titanium trichloride-sulfuric acid reagent R.
Allow to stand for 30 min and filter. The absorbance (2.2.25) of the filtrate, measured at 405 nm using a mixture of 25 mL of a filtered 40 g/L suspension of the substance to be examined and 2 mL of a 13 per cent V/V solution of sulfuric acid R as the compensation liquid, has a maximum of 0.35.
For type B use 10 mL of the suspension and dilute to 25 mL with water R for the test.
Water-soluble substances
Maximum 1.5 per cent.
Place 25.0 g in a 400 mL beaker, add 200 mL of water R and stir for 1 h using a magnetic stirrer. Transfer the suspension to a 250.0 mL volumetric flask, rinsing with water R, and dilute to volume with the same solvent. Allow the bulk of the solids to settle. Filter about 100 mL of the almost clear supernatant through a membrane filter (nominal pore size 0.45 μm), protected by superimposing a membrane filter (nominal pore size 3 μm). While filtering, stir the liquid above the membrane filter manually or by means of a mechanical stirrer, taking care not to damage the membrane filter. Transfer 50.0 mL of the clear filtrate to a tared 100 mL beaker, evaporate to dryness and dry at 105-110 °C for 3 h. The residue weighs a maximum of 75 mg.
Impurity A
Liquid chromatography (2.2.29).
Test solution: Suspend 1.250 g in 50.0 mL of methanol R and shake for 60 min. Leave the bulk to settle and filter through a membrane filter (nominal pore size 0.2 μm).
Reference solution (a): Dissolve 50 mg of 1-vinylpyrrolidin-2-one R (impurity A) in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with methanol R. Dilute 5.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution (b): Dissolve 10 mg of 1-vinylpyrrolidin-2-one R (impurity A) and 0.50 g of vinyl acetate R in methanol R and dilute to 100 mL with the same solvent. Dilute 1 mL of the solution to 100 mL with the mobile phase.
Precolumn:
— size: l = 0.025 m, Ø = 4 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Column:
— size: l = 0.25 m, Ø = 4 mm;
— stationary phase: polar end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 40 °C.
Mobile phase acetonitrile for chromatography R, water for chromatography R (10:90 V/V).
Flow rate 1 mL/min.
Detection: Spectrophotometer at 235 nm.
Injection 50 μL. After each injection of the test solution, wash the precolumn by passing the mobile phase backwards, at the same flow rate as applied in the test, for 30 min.
System suitability:
— resolution: minimum 2.0 between the peaks due to impurity A and vinyl acetate in the chromatogram obtained with reference solution (b);
— repeatability: maximum relative standard deviation of 2.0 per cent determined on 6 injections of reference
solution (a).
Calculation of percentage content:
— for impurity A, use the concentration of impurity A in reference solution (a).
Limit:
— impurity A: maximum 10 ppm.
Loss on drying (2.2.32)
Maximum 5.0 per cent, determined on 0.500 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Place 0.100 g of the substance to be examined (m mg) in a combustion flask and add 5 g of a mixture of 1 g of copper sulfate pentahydrate R, 1 g of titanium dioxide R and 33 g of dipotassium sulfate R, and 3 glass beads. Wash any adhering particles from the neck into the flask with a small quantity of water R. Add 7 mL of sulfuric acid R, allowing it to run down the inside wall of the flask. Gradually heat the flask until the solution has a clear, yellowish-green colour, and the inside wall of the flask is free from carbonised material, and then heat for a further 45 min. After cooling, cautiously add 20 mL of water R, and connect the flask to the distillation apparatus, which has been previously washed by passing steam through it. To the absorption flask add 30 mL of a 40 g/L solution of boric acid R, 0.15 mL of bromocresol green-methyl red solution R and sufficient water R to immerse the lower end of the condenser tube. Add 30 mL of strong sodium hydroxide solution R through a funnel, cautiously rinse the funnel with 10 mL of water R, immediately close the clamp attached to the rubber tube, then start the distillation with steam to obtain 80-100 mL of distillate. Remove the absorption flask from the lower end of the condenser tube, rinsing the end part with a small quantity of water R, and titrate the distillate with 0.025 M sulfuric acid until the colour of the solution changes from green through pale greyish-blue to pale greyish red-purple. Carry out a blank determination and make any necessary correction.
1 mL of 0.025 M sulfuric acid is equivalent to 0.700 mg of N.
STORAGE
In an airtight container.
♦ LABELLING
The label states the type of crospovidone (type A or type B).♦
IMPURITIES
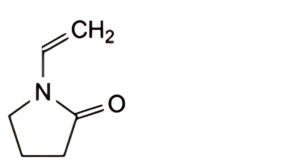
A. 1-ethenylpyrrolidin-2-one (1-vinylpyrrolidin-2-one).
♢ FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for crospovidone used as disintegrant.
Hydration capacity
Introduce 2.0 g into a 100 mL centrifuge tube and add 40 mL of water R. Shake vigorously until a suspension is obtained. Shake again 5 min and 10 min later, then centrifuge for 15 min at 750 g. Decant the supernatant and weigh the residue. The hydration capacity is the ratio of the mass of the residue to the initial mass of the sample. It is typically 3 to 9.
Particle-size distribution (2.9.31)
Powder flow (2.9.36)
The following characteristic may be relevant for crospovidone used as suspension stabiliser.
Settling volume
Introduce 10 g into a 100 mL graduated cylinder and add 90 mL of water R. Shake vigorously. Dilute to 100 mL with
water R, washing the powder residues from the walls of the cylinder. Allow to stand for 24 h, then read the volume of the sediment. It is typically greater than 60 mL.♢