Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Prostaglandin (PGF2α) analogue.
Preparation
Cloprostenol Injection
DEFINITION
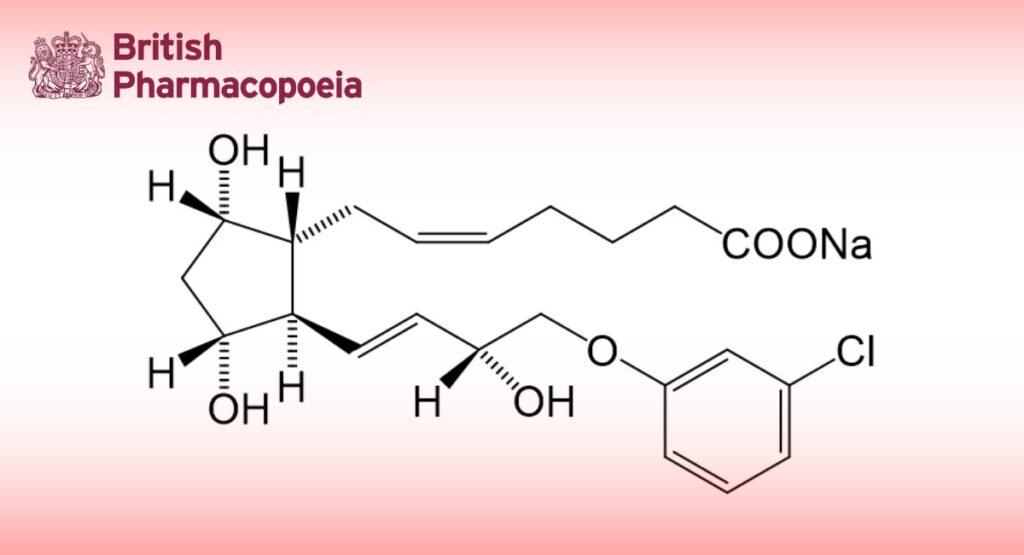
Cloprostenol Sodium is (±)-(5Z)-7-(1R,3R,5S)-2-[(1E,3R)-4-(3-chlorophenoxy)-3-hydroxybut-1-enyl]-3,5- dihydroxycyclopentylhept-5-enoate. It contains not less than 97.5% and not more than 102.5% of C22H28ClNaO6, calculated with reference to the anhydrous substance.
CAUTION Cloprostenol Sodium is extremely potent and extraordinary care should be taken in any procedure in which it is used.
CHARACTERISTICS
A white or almost white, amorphous powder; hygroscopic.
Freely soluble in water, in ethanol (96%) and in methanol; practically insoluble in acetone.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of cloprostenol sodium (RSV 11).
B. Yields reaction A characteristic of sodium salts, Appendix VI.
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in absolute ethanol.
(1) 2.0% w/v of the substance being examined.
(2) 0.050% w/v of the substance being examined.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with silica gel for chromatography (5 µm) (Partisil is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.8 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 5 µL of each solution.
(g) Allow the chromatography to proceed for twice the retention time of the peak due to Cloprostenol.
MOBILE PHASE
1 volume of glacial acetic acid, 70 volumes of absolute ethanol and 930 volumes of hexane.
LIMITS
In the chromatogram obtained with solution (1):
the sum of the areas of any secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2.5%).
Water
Not more than 3.0% w/w, Appendix IX C. Use 50 mg dissolved in 1 mL of absolute ethanol.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in absolute ethanol.
(1) 0.08% w/v of the substance being examined.
(2) 0.08% w/v of cloprostenol sodium BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with silica gel for chromatography (5 µm) (Partisil is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.8 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 5 µL of each solution.
MOBILE PHASE
1 volume of glacial acetic acid, 100 volumes of absolute ethanol and 900 volumes of hexane.
DETERMINATION OF CONTENT
Calculate the content of C22H28ClNaO6 from the chromatograms obtained and using the declared content of C22H28ClNaO6 in cloprostenol sodium BPCRS.
STORAGE
Cloprostenol Sodium should be protected from light and moisture.
IMPURITIES
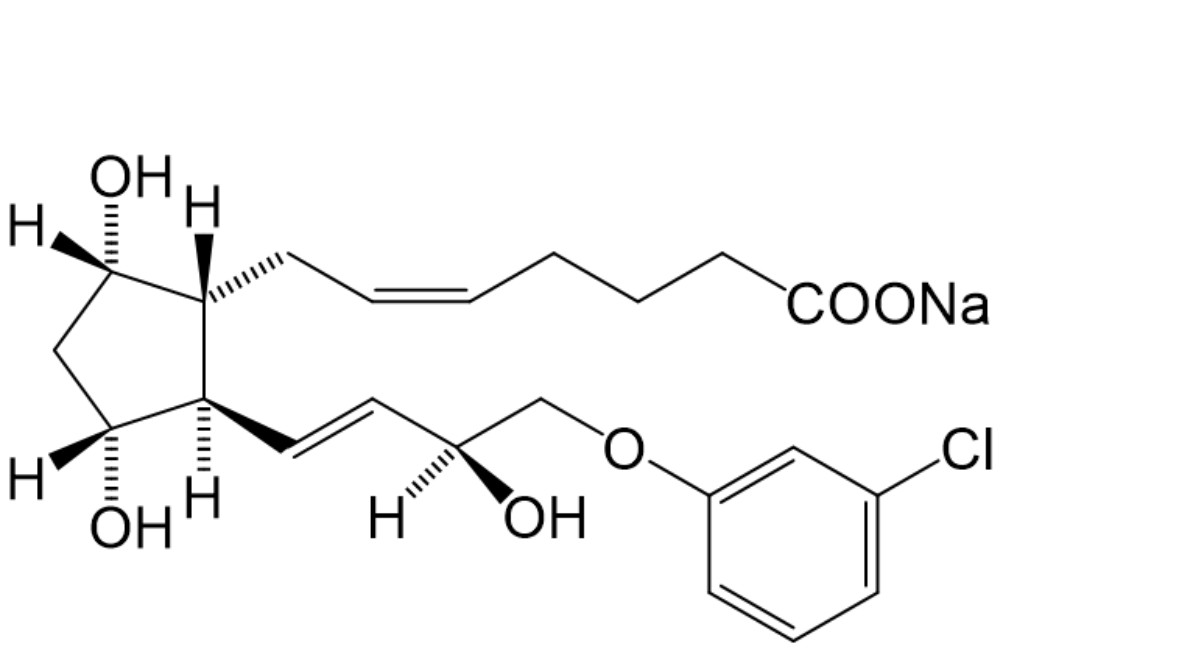
A. (±)-(5Z )-7-(1R,3R,5S)-2-[(1E,3S)-4-(3-chlorophenoxy)-3-hydroxybut-1-enyl]-3,5-dihydroxycyclopentylhept-5-enoate (epimer),
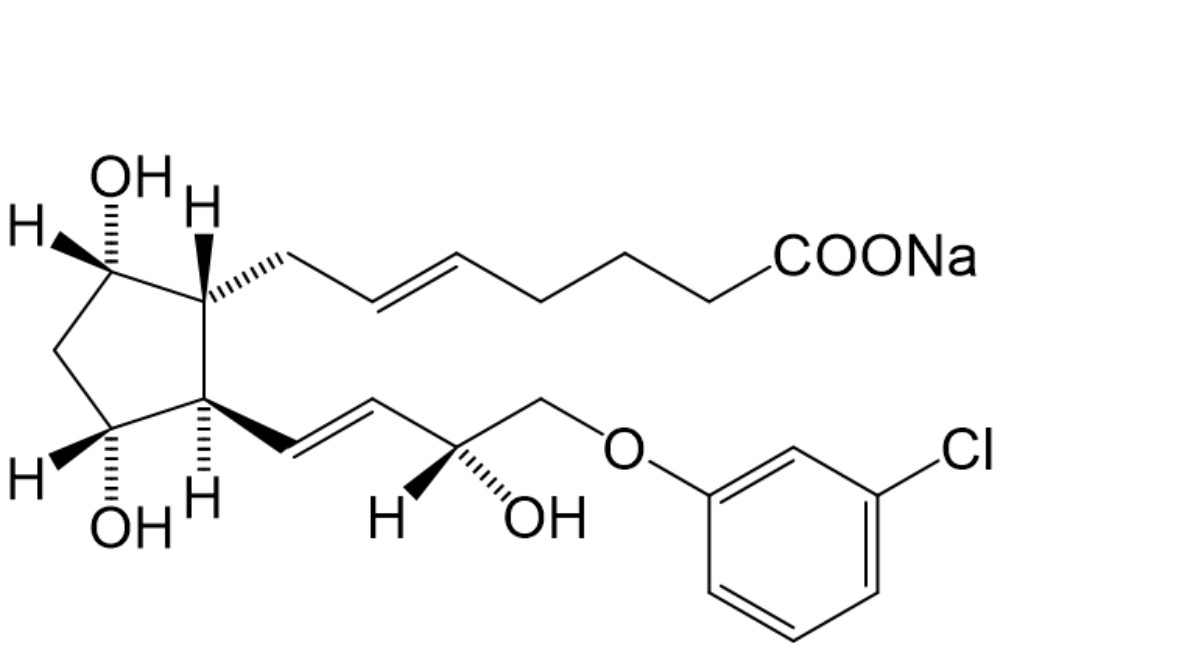
B. (±)-(5E )-7-(1R,3R,5S)-2-[(1E,3 R)-4-(3-chlorophenoxy)-3-hydroxybut-1-enyl]-3,5-dihydroxycyclopentylhept-5-enoate (trans-isomer).