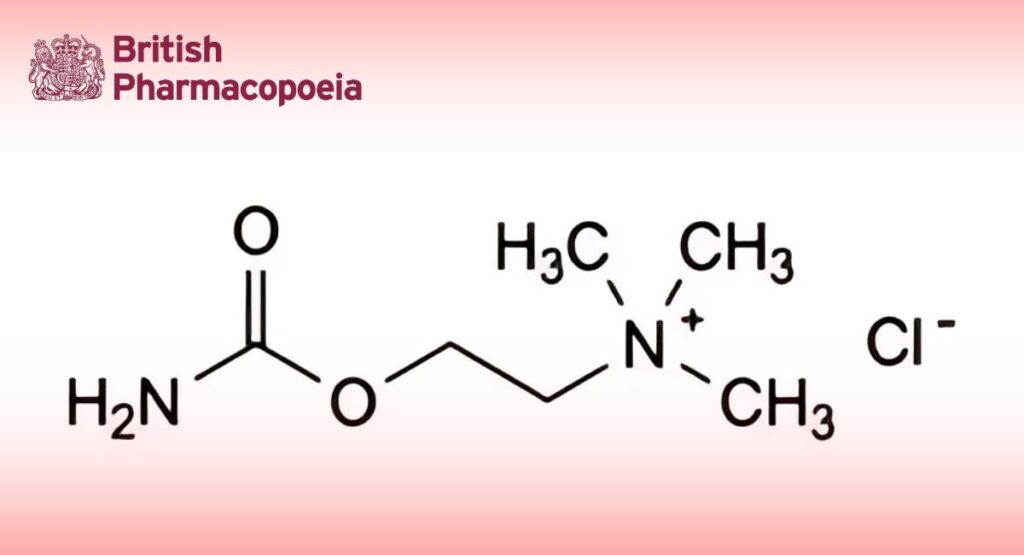
(Ph. Eur. monograph 1971)
C6H15ClN2O2 182.7 51-83-2
Action and use
Cholinoceptor agonist.
DEFINITION
2-(Carbamoyloxy)-N,N,N-trimethylethanaminium chloride.
Content
99.0 per cent to 101.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline, hygroscopic powder.
Solubility
Very soluble in water, sparingly soluble in ethanol (96 per cent), practically insoluble in acetone.
IDENTIFICATION
First identification: A, C.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: carbachol CRS.
B. Examine the chromatograms obtained in the test for related substances.
Results: The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. 0.5 mL of solution S (see Tests) gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 2.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 2.0 mL of solution S, add 0.05 mL of methyl red mixed solution R. Not more than 0.2 mL of 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the colour of the indicator.
Related substances
Thin-layer chromatography (2.2.27).
Prepare the solutions immediately before use.
Test solution (a): Dissolve 0.20 g of the substance to be examined in methanol R and dilute to 5.0 mL with the same solvent.
Test solution (b): Dilute 2.0 mL of test solution (a) to 20.0 mL with methanol R.
Reference solution (a): Dissolve 20 mg of carbachol CRS in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution (b): Dissolve 8 mg of choline chloride R and 8 mg of acetylcholine chloride CRS in methanol R and dilute to 10.0 mL with the same solvent. Dilute 5.0 mL to 10.0 mL with methanol R.
Plate: cellulose for chromatography R as the coating substance.
Mobile phase: water R, methanol R (10:90 V/V).
Application: 10 μL.
Development: Over 2/3 of the plate.
Detection: Spray with potassium iodobismuthate solution R3.
System suitability: The chromatogram obtained with reference solution (b) shows 2 clearly separated spots.
Limits: In the chromatogram obtained with test solution (a):
— any impurity: any spot, apart from the principal spot, is not more intense than one or other of the 2 principal spots in the chromatogram obtained with reference solution (b) (1 per cent). Compare the spots with the spot of the most appropriate colour in the chromatogram obtained with reference solution (b).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g of the residue obtained in the test for loss on drying.
ASSAY
Dissolve 0.150 g in a mixture of 10 mL of anhydrous acetic acid R and 40 mL of acetic anhydride R. Titrate with 0.1 M perchloric acid. Determine the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 18.27 mg of C6H15ClN2O2.
STORAGE
In an airtight container, protected from light.
IMPURITIES
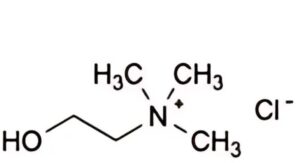
A. 2-hydroxy-N,N,N-trimethylethanaminium chloride (choline chloride).