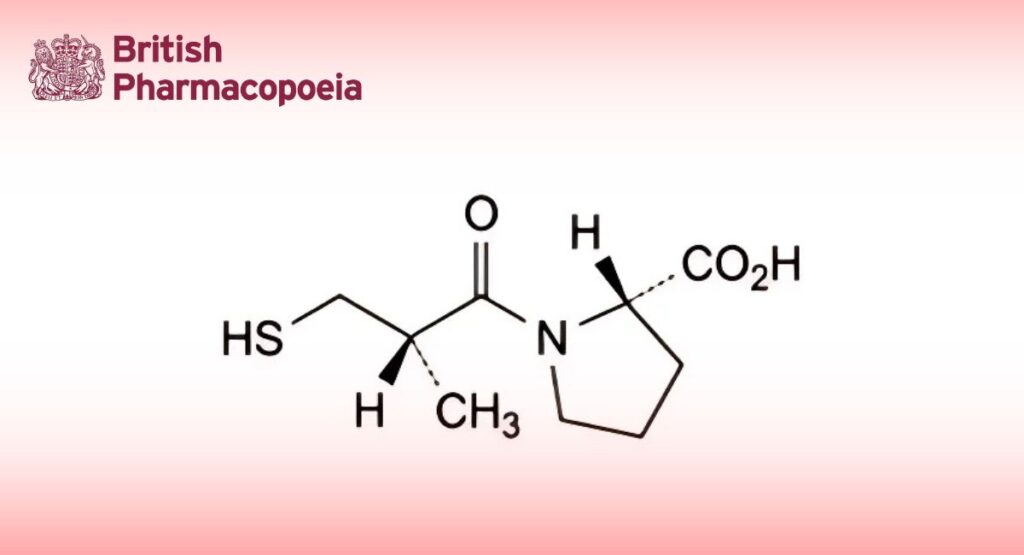
(Ph. Eur. monograph 1079)
C9H15NO3S 217.3 62571-86-2
Action and use
Angiotensin converting enzyme inhibitor.
Preparations
Captopril Oral Solution
Captopril Tablets
DEFINITION
(2S)-1-[(2S)-2-Methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic acid.
Content
98.0 per cent to 101.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Soluble in water, freely soluble in methanol and in methylene chloride. It dissolves in dilute solutions of alkali hydroxides.
IDENTIFICATION
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: captopril CRS.
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
pH (2.2.3)
2.0 to 2.6 for solution S.
Specific optical rotation (2.2.7)
-132 to -127 (dried substance).
Dissolve 0.250 g in anhydrous ethanol R and dilute to 25.0 mL with the same solvent.
Impurity F
Gas chromatography (2.2.28).
Reagent solution: Add 2.8 mL of acetyl chloride R dropwise to 17.2 mL of anhydrous methanol R at 0 °C and mix. Allow to stand for 20 min at room temperature before use.
Test solution: Introduce 20.0 mg of the substance to be examined into a vial and add 1.0 mL of the reagent solution. Mix and heat at 60 °C for 30 min.
Evaporate to dryness under a stream of nitrogen R. Dissolve the residue in 0.5 mL of ethyl acetate R, add 0.5 mL of pentafluoropropionic anhydride R, mix and heat at 60 °C for 30 min. Evaporate to dryness under a stream of nitrogen R. Dissolve the residue in 1.0 mL of butyl acetate R.
Reference solution (a): Dissolve the contents of a vial of captopril for system suitability CRS (containing impurity F) in 1 mL of the reagent solution. Prepare as described for the test solution.
Reference solution (b): Mix 0.25 mL of reference solution (a) and 0.75 mL of butyl acetate R.
Column:
— material: fused silica;
— size: l = 25 m, Ø = 0.32 mm;
— stationary phase: phenyl(5)methyl(95)polysiloxane R (film thickness 1 μm).
Carrier gas helium for chromatography R.
Flow rate: 1.2 mL/min.
Split ratio: 1:20.
Temperature:
| Time
(min) |
Temperature
(°C) |
|
| Column | 0 – 10 | 200 |
| 10 – 14 | 200 → 240 | |
| 14 – 34 | 240 | |
| Injection port | 270 | |
| Detector | 300 |
Injection port: 270
Detector: 300
Detection: Flame ionisation.
Injection: 1 μL.
Relative retention: With reference to captopril (retention time = about 6 min): impurity F = about 0.96.
System suitability:
— resolution: minimum 1.5 between the peaks due to impurity F and captopril in the chromatogram obtained with reference solution (a);
— signal-to-noise ratio: minimum 10 for the peak due to impurity F in the chromatogram obtained with reference solution (b).
Calculate the percentage content of impurity F using the following expression:
A/(A+B) × 100
A = area of the peak due to impurity F in the chromatogram obtained with the test solution;
B = area of the peak due to captopril in the chromatogram obtained with the test solution.
Limit:
— impurity F: maximum 0.2 per cent.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture phosphoric acid R, acetonitrile R1, water R (0.08:10:90 V/V/V).
Test solution: Dissolve 0.125 g of the substance to be examined in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution (a): Dissolve 4.0 mg of captopril impurity J CRS, 5.0 mg of captopril impurity B CRS, 5.0 mg of captopril impurity C CRS and 5.0 mg of captopril impurity D CRS in the solvent mixture and dilute to 50.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 20.0 mL with the solvent mixture. Prepare immediately before use.
Reference solution (b): Dissolve 5 mg of the substance to be examined and 5 mg of captopril impurity E CRS in
acetonitrile R and dilute to 25 mL with the same solvent. Dilute 4 mL of the solution to 50 mL with the solvent mixture.
Reference solution (c): In order to prepare impurity A in situ, introduce 1.0 mL of the test solution into a volumetric flask and add 230 μL of 0.05 M iodine. If the solution is not colourless, add 0.1 M sodium thiosulfate dropwise until it becomes colourless, and dilute to 50 mL with the solvent mixture. Dilute 5 mL of this solution to 20 mL with the solvent mixture.
Reference solution (d): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Column:
— size: l = 0.3 m, Ø = 3.9 mm;
— stationary phase: irregular end-capped octadecylsilyl silica gel for chromatography R (10 μm);
— temperature: 50 °C.
Mobile phase:
— mobile phase A: phosphoric acid R, water for chromatography R (0.08:100 V/V);
— mobile phase B: phosphoric acid R, acetonitrile R1, water for chromatography R (0.08:50:50 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 5 | 90 | 10 |
| 5 – 20 | 90 → 50 | 10 → 50 |
| 20 – 45 | 50 | 50 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 210 nm.
Injection: 25 μL.
Identification of impurities: Use the chromatogram obtained with reference solution (a) to identify the peaks due to impurities B, C, D and J; use the chromatogram obtained with reference solution (b) to identify the peak due to impurity E; use the chromatogram obtained with reference solution (c) to identify the peak due to impurity A.
Relative retention: With reference to captopril (retention time = about 15 min): impurity C = about 0.6; impurity D = about 0.8; impurity E = about 0.9; impurity B = about 1.17; impurity J = about 1.22; impurity A = about 1.7.
System suitability:
— resolution: minimum 1.5 between the peaks due to impurities B and J in the chromatogram obtained with reference solution (a);
— resolution: minimum 2.0 between the peaks due to impurity E and captopril in the chromatogram obtained with reference solution (b).
Limits:
— impurity A: not more than 10 times the area of the principal peak in the chromatogram obtained with reference solution (d) (1.0 per cent);
— impurity J: not more than 2.5 times the area of the corresponding peak in the chromatogram obtained with
reference solution (a) (0.2 per cent);
— impurities B, C, D: for each impurity, not more than 1.5 times the area of the corresponding peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
— impurity E: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (d) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (d) (0.10 per cent);
— total: maximum 1.2 per cent;
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (d) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in vacuo at 60 °C at a pressure not exceeding 0.1 kPa for 3 h.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.150 g in 30 mL of water R. Titrate with 0.05 M iodine, determining the end-point potentiometrically (2.2.20). Use a combined platinum electrode.
1 mL of 0.05 M iodine is equivalent to 21.73 mg of C9H15NO3S.
IMPURITIES
Specified impurities A, B, C, D, E, F, J.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) G, H, I, L, M, N, O.
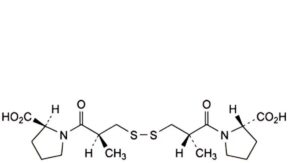
A. 1,1′-[disulfanediylbis[(2S)-2-methyl-1-oxopropane-3,1-diyl]]bis[(2S)-pyrrolidine-2-carboxylic] acid (captopril disulfide),
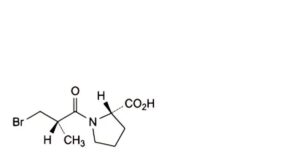
B. (2S)-1-[(2S)-3-bromo-2-methylpropanoyl]pyrrolidine-2-carboxylic acid,
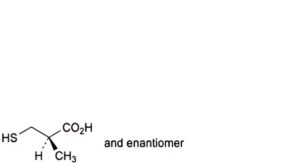
C. (2RS)-2-methyl-3-sulfanylpropanoic acid,
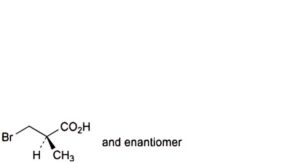
D. (2RS)-3-bromo-2-methylpropanoic acid,
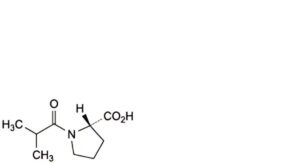
E. (2S)-1-(2-methylpropanoyl)pyrrolidine-2-carboxylic acid,
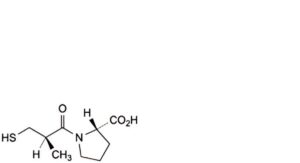
F. (2S)-1-[(2R)-2-methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic acid (epi-captopril),
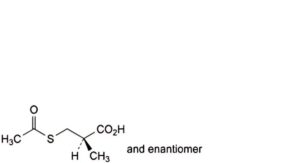
G. (2RS)-3-(acetylsulfanyl)-2-methylpropanoic acid,
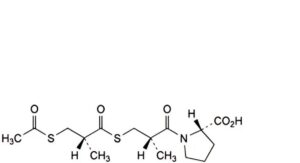
H. (2S)-1-[(2S)-3-[[(2R)-3-(acetylsulfanyl)-2-methylpropanoyl]sulfanyl]-2-methylpropanoyl]pyrrolidine-2-carboxylic acid,
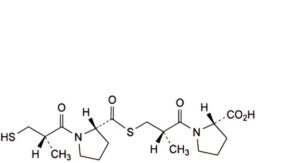
I. (2S)-1-[(2S)-3-[[[(2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolidin-2-yl]carbonyl]sulfanyl]-2-methylpropanoyl]pyrrolidine-2-carboxylic acid,
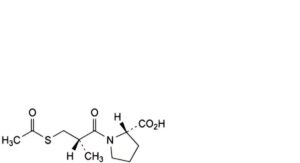
J. (2S)-1-[(2S)-3-(acetylsulfanyl)-2-methylpropanoyl]pyrrolidine-2-carboxylic acid (acetylcaptopril),
L. 1,1′-[methylenebis[sulfanediyl[(2S)-2-methyl-1-oxopropane-3,1-diyl]]]bis[(2S)-pyrrolidine-2-carboxylic] acid,
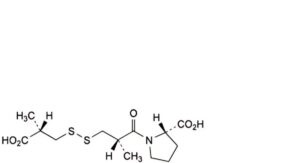
M. (2S)-1-[(2S)-3-[[(2S)-2-carboxypropyl]disulfanyl]-2-methylpropanoyl]pyrrolidine-2-carboxylic acid,
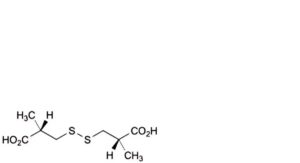
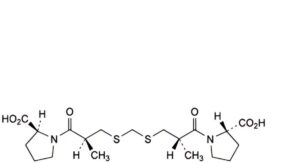
N. 3,3′-disulfanediylbis[(2S)-2-methylpropanoic] acid,
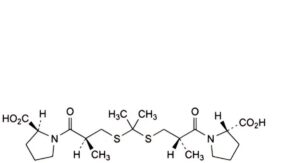
O. 1,1′-[propane-2,2-diylbis[sulfanediyl[(2S)-2-methyl-1-oxopropane-3,1-diyl]]]bis[(2S)-pyrrolidine-2-carboxylic] acid.