(Ph. Eur. monograph 1296)
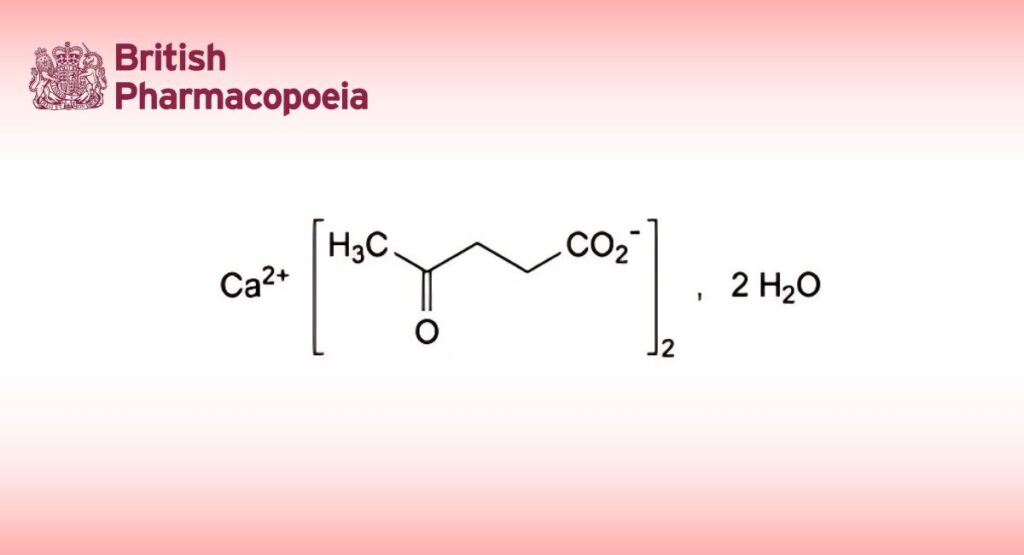
C10H14CaO6,2H2O 306.3 5743-49-7
Action and use
Source of calcium.
DEFINITION
Calcium di(4-oxopentanoate) dihydrate.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, very slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: A, D, E.
Second identification: B, C, D, E.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison calcium levulinate dihydrate CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 60 mg of the substance to be examined in water R and dilute to 1 mL with the same solvent.
Reference solution: Dissolve 60 mg of calcium levulinate dihydrate CRS in water R and dilute to 1 mL with the same solvent.
Plate: TLC silica gel plate R.
Mobile phase concentrated ammonia R, ethyl acetate R, water R, ethanol (96 per cent) R (10:10:30:50 V/V/V/V).
Application: 10 μL.
Development: Over a path of 10 cm.
Drying: At 100-105 °C for 20 min and allow to cool.
Detection: Spray with a 30 g/L solution of potassium permanganate R. Dry in a current of warm air for about 5 min or until the spots become yellow. Examine in daylight.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. To 1 mL of solution S (see Tests), add 20 mL of a 2.5 g/L solution of dinitrophenylhydrazine R in dilute hydrochloric acid R. Allow to stand for 15 min. Filter, wash the precipitate with water R. Dry the precipitate in an oven at 100-105 °C. The melting point (2.2.14) is 203 °C to 210 °C.
D. It gives reaction (b) of calcium (2.3.1).
E. Loss on drying (see Tests).
TESTS
Solution S
Dissolve 10.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
pH (2.2.3)
6.8 to 7.8 for solution S.
Oxidisable substances
To 1 mL of solution S, add 10 mL of water R, 1 mL of dilute sulfuric acid R and 0.25 mL of a 3.0 g/L solution of potassium permanganate R. Mix. After 5 min, the violet colour of the mixture is still visible.
Sucrose and reducing sugars
To 5 mL of solution S add 2 mL of hydrochloric acid R1 and dilute to 10 mL with water R. Heat to boiling for 5 min and allow to cool. Add 10 mL of sodium carbonate solution R. Allow to stand for 5 min, dilute to 25 mL with water R and filter. To 5 mL of the filtrate add 2 mL of cupri-tartaric solution R and heat to boiling for 1 min. No red precipitate is formed.
Chlorides (2.4.4)
Maximum 50 ppm.
Dilute 10 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 200 ppm.
Dilute 7.5 mL of solution S to 15 mL with distilled water R.
Magnesium and alkali metals
Maximum 1.0 per cent.
To 10 mL of solution S, add 80 mL of water R, 10 mL of ammonium chloride solution R and 1 mL of ammonia R. Heat to boiling. To the boiling solution, add dropwise 50 mL of warm ammonium oxalate solution R. Allow to stand for 4 h, then dilute to 200 mL with water R and filter. To 100 mL of the filtrate, add 0.5 mL of sulfuric acid R. Evaporate to dryness on a water-bath and ignite to constant mass at 600 ± 50 °C. The residue weighs a maximum of 5.0 mg.
Loss on drying (2.2.32)
11.0 per cent to 12.5 per cent, determined on 0.200 g by drying at 105 °C.
Pyrogens (2.6.8)
If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of pyrogens, it complies with the test for pyrogens. Inject per kilogram of the rabbit’s mass 4 mL of a solution containing per millilitre 50 mg of the substance to be examined.
ASSAY
Dissolve 0.240 g in 50 mL of water R. Carry out the complexometric titration of calcium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 27.03 mg of C10H14CaO6.
STORAGE
Protected from light.