(Ph. Eur. monograph 0980)
C3H7CaO6P,xH2O 210.1 (anhydrous substance)
Anhydrous calcium glycerophosphate 27214-00-2
Action and use
Excipient.
DEFINITION
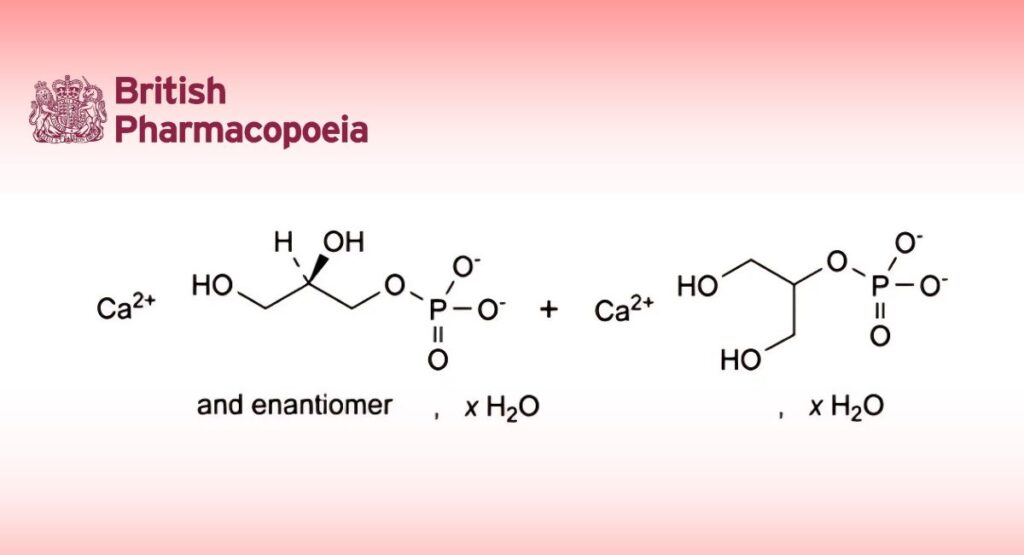
Mixture in variable proportions of the calcium salt of (2RS)-2,3-dihydroxypropyl phosphate and of 1,3-dihydroxypropan-2-yl phosphate. It may be anhydrous or contain a variable quantity of water.
Content
18.6 per cent to 19.4 per cent of Ca (dried substance).
CHARACTERS
Appearance
White or almost white powder, hygroscopic.
Solubility
Sparingly soluble in water, practically insoluble in ethanol (96 per cent).
IDENTIFICATION
A. Mix 1 g with 1 g of potassium hydrogen sulfate R in a test tube fitted with a glass tube. Heat strongly and direct the white vapour towards a piece of filter paper impregnated with a freshly prepared 10 g/L solution of sodium nitroprusside R. The filter paper develops a blue colour in contact with piperidine R.
B. Ignite 0.1 g in a crucible. Take up the residue with 5 mL of nitric acid R and heat on a water-bath for 1 min. Filter. The filtrate gives reaction (b) of phosphates (2.3.1).
C. It gives reaction (b) of calcium (2.3.1).
TESTS
Solution S
Dissolve 1.5 g at room temperature in carbon dioxide-free water R prepared from distilled water R and dilute to 150 mL with the same solvent.
Appearance of solution
Solution S is not more opalescent than reference suspension III (2.2.1).
Acidity or alkalinity
To 100 mL of solution S add 0.1 mL of phenolphthalein solution R. Not more than 1.5 mL of 0.1 M hydrochloric acid or 0.5 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator.
Citric acid
Shake 5.0 g with 20 mL of carbon dioxide-free water R and filter. To the filtrate add 0.15 mL of sulfuric acid R and filter again. To the filtrate add 5 mL of mercuric sulfate solution R and heat to boiling. Add 0.5 mL of a 3.2 g/L solution of potassium permanganate R and again heat to boiling. No precipitate is formed.
Glycerol and ethanol (96 per cent)-soluble substances
Maximum 0.5 per cent.
Shake 1.000 g with 25 mL of ethanol (96 per cent) R for 1 min. Filter. Evaporate the filtrate on a water-bath and dry the residue at 70 °C for 1 h. The residue weighs a maximum of 5 mg.
Chlorides (2.4.4)
Maximum 500 ppm.
Dissolve 0.1 g in a mixture of 2 mL of acetic acid R and 8 mL of water R and dilute to 15 mL with water R.
Phosphates (2.4.11)
Maximum 400 ppm.
Dilute 2.5 mL of solution S to 100 mL with water R.
Sulfates (2.4.13)
Maximum 0.1 per cent, determined on solution S.
Iron (2.4.9)
Maximum 50 ppm, determined on 0.20 g.
Loss on drying (2.2.32)
Maximum 12.0 per cent, determined on 1.000 g by drying in an oven at 150 °C for 4 h.
ASSAY
Dissolve 0.200 g in water R. Carry out the complexometric titration of calcium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 4.008 mg of Ca.