(Ph. Eur. monograph 0172)
C12H22CaO14,H2O 448.4 18016-24-5
Action and use
Used in treatment of calcium deficiency.
Preparations
Calcium Gluconate Tablets
Calcium Gluconate Chewable Tablets
Calcium Gluconate Effervescent Tablets
DEFINITION
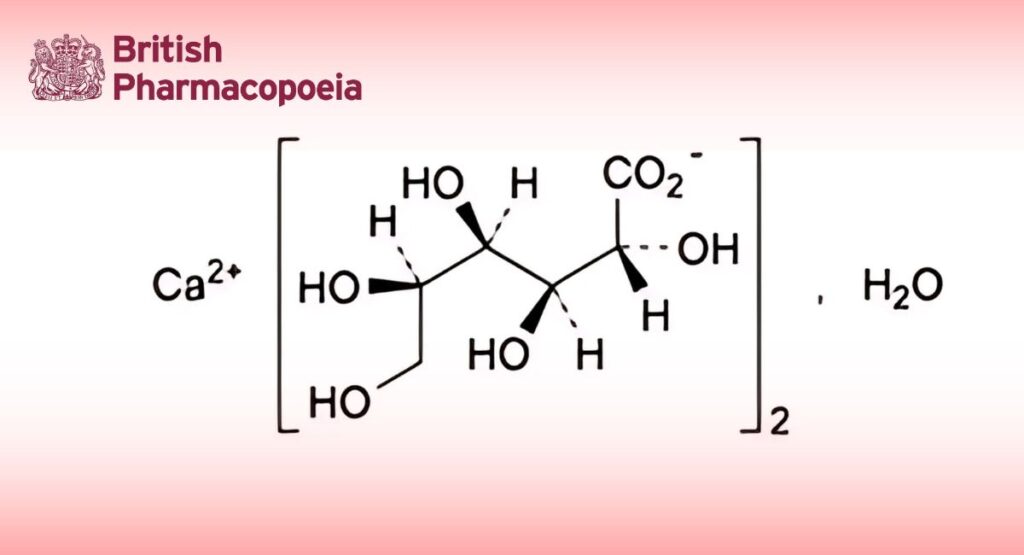
Calcium bis[(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate] monohydrate (calcium di(D-gluconate) monohydrate).
Content
98.5 per cent to 102.0 per cent of C12H22CaO14,H2O.
CHARACTERS
Appearance
White or almost white, crystalline or granular powder.
Solubility
Sparingly soluble in water, freely soluble in boiling water.
Calcium Gluconate
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 20 mg of the substance to be examined in 1 mL of water R, heating if necessary in a water-bath at 60 °C.
Reference solution: Dissolve 20 mg of calcium gluconate CRS in 1 mL of water R, heating if necessary in a water-bath at 60 °C.
Plate: TLC silica gel plate R (5-40 μm) [or TLC silica gel plate R (2-10 μm)].
Mobile phase: concentrated ammonia R, ethyl acetate R, water R, ethanol (96 per cent) R (10:10:30:50 V/V/V/V).
Application: 1 μL.
Development: Over 2/3 of the plate.
Drying: At 100 °C for 20 min; allow to cool.
Detection: Spray with a solution containing 10 g/L of cerium sulfate R and 25 g/L of ammonium molybdate R in dilute sulfuric acid R and heat at 105 °C for about 10 min.
Results: After 5 min, the principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
B. Solution S (see Tests) gives reaction (b) of calcium (2.3.1).
TESTS
Solution S
Dissolve 1.0 g in water R heated to 60 °C and dilute to 50 mL with the same solvent.
Appearance of solution
At 60 °C, solution S is not more intensely coloured than reference solution Y6 (2.2.2, Method II). After cooling, it is not more opalescent than reference suspension II (2.2.1).
Organic impurities and boric acid
Introduce 0.5 g into a porcelain dish previously rinsed with sulfuric acid R and placed in a bath of iced water. Add 2 mL of cooled sulfuric acid R and mix. No yellow or brown colour develops. Add 1 mL of chromotrope II B solution R. A violet colour develops and does not become dark blue. The solution is not more intensely coloured than that of a mixture of 1 mL of chromotrope II B solution R and 2 mL of cooled sulfuric acid R.
Sucrose and reducing sugars
Dissolve 0.5 g in a mixture of 2 mL of hydrochloric acid R1 and 10 mL of water R. Boil for 5 min, allow to cool, add 10 mL of sodium carbonate solution R and allow to stand. Dilute to 25 mL with water R and filter. To 5 mL of the filtrate add 2 mL of cupri-tartaric solution R and boil for 1 min. Allow to stand for 2 min. No red precipitate is formed.
Chlorides (2.4.4)
Maximum 200 ppm.
Dilute 12.5 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 100 ppm.
Dissolve 10.0 g with heating in a mixture of 10 mL of acetic acid R and 90 mL of distilled water R.
Magnesium and alkali metals
Maximum 0.4 per cent.
Dissolve 1.00 g in 100 mL of boiling water R, add 10 mL of ammonium chloride solution R, 1 mL of ammonia R and, dropwise, 50 mL of hot ammonium oxalate solution R. Allow to stand for 4 h, dilute to 200 mL with water R and filter. Evaporate 100 mL of the filtrate to dryness and ignite. The residue weighs a maximum of 2 mg.
Microbial contamination
TAMC: acceptance criterion 10 CFU/g (2.6.12).
TYMC: acceptance criterion 10 CFU/g (2.6.12).
ASSAY
Dissolve 0.8000 g in 20 mL of hot water R, allow to cool and dilute to 300 mL with water R. Carry out the complexometric titration of calcium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 44.84 mg of C12H22CaO14,H2O.