Calcium Folinate
(Ph. Eur. monograph 0978)
C20H21CaN7O7,xH2O 511.5 (anhydrous substance) 2060570-47-8
Action and use
Antidote to folic acid antagonists.
Preparations
Calcium Folinate Injection
Calcium Folinate Tablets
DEFINITION
Calcium (2S)-2-[4-[[[(6RS)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6 yl]methyl]amino]benzamido]pentanedioate hydrate.
Content
— calcium folinate (C20H21CaN7O7; Mr 511.5): 97.0 per cent to 102.0 per cent (anhydrous substance)
— calcium (Ca; Ar 40.08): 7.54 per cent to 8.14 per cent (anhydrous substance).
It contains a variable quantity of water.
CHARACTERS
Appearance
White or light yellow, amorphous or crystalline, hygroscopic powder.
Solubility
Sparingly soluble in water, practically insoluble in acetone and in ethanol (96 per cent).
The amorphous form may produce supersaturated solutions in water.
It shows polymorphism (5.9).
IDENTIFICATION
First identification: A, B, D.
Second identification: A, C, D.
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison calcium folinate CRS.
If the spectra obtained show differences, dissolve the substance to be examined and the reference substance separately in the minimum volume of water R and add dropwise sufficient acetone R to produce a precipitate. Allow to stand for 15 min, collect the precipitate by centrifugation, wash the precipitate with 2 small quantities of acetone R and dry. Record new spectra using the residues.
C. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 15 mg of the substance to be examined in a 3 per cent V/V solution of ammonia R and dilute to 5 mL with the same solvent.
Reference solution: Dissolve 15 mg of calcium folinate CRS in a 3 per cent V/V solution of ammonia R and dilute to 5 mL with the same solvent.
Plate cellulose for chromatography F254 R as the coating substance.
Mobile phase: The lower layer of a mixture of 1 volume of isoamyl alcohol R and 10 volumes of a 50 g/L solution of citric acid monohydrate R previously adjusted to pH 8 with ammonia R.
Application 5 μL.
Development: Over 2/3 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
D. It gives reaction (b) of calcium (2.3.1).
TESTS
Carry out the tests as rapidly as possible, protected from actinic light.
Solution S
Dissolve 1.25 g in carbon dioxide-free water R, heating at 40 °C if necessary, and dilute to 50.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1).
pH (2.2.3)
6.8 to 8.0 for solution S.
Specific optical rotation (2.2.7)
+ 14.4 to + 18.0 (anhydrous substance), determined on solution S.
Absorbance (2.2.25)
Maximum 0.60, determined at 420 nm on solution S.
Ethanol
Head-space gas chromatography (2.2.28): use the standard additions method.
Test solution Dissolve 0.25 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution Dilute 0.750 g of anhydrous ethanol R to 1000.0 mL with water R.
Column:
— material: fused silica;
— size: l = 10 m, Ø = 0.32 mm;
— stationary phase: styrene-divinylbenzene copolymer R.
Carrier gas: nitrogen for chromatography R.
Flow rate: 4 mL/min.
Static head-space conditions that may be used:
— equilibration temperature: 80 °C;
— equilibration time: 20 min;
— pressurisation time: 30 s.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 6 | 125 → 185 |
| 6 – 15 | 185 | |
| Injection port | 250 | |
| Detector | 250 |
Detection: Flame ionisation.
Injection: At least 3 times.
Limit:
— ethanol: maximum 3.0 per cent.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution: Dissolve 10.0 mg of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 10.0 mg of calcium folinate CRS in water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
Reference solution (c): Dissolve 5 mg of calcium folinate for system suitability A CRS (containing impurities A, B, C, D, E, F and I) in 5 mL of a 2.5 g/L solution of sodium hydrogen carbonate R.
Column:
— size: l = 0.25 m, Ø = 4 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 40 °C.
Mobile phase: Mix 165 mL of methanol R and 835 mL of a solution containing 4.0 mL of tetrabutylammonium dihydrogen phosphate solution R and 1.42 g of disodium hydrogen phosphate dihydrate R in water for chromatography R, previously adjusted to pH 7.7 with phosphoric acid R or dilute sodium hydroxide solution R.
Flow rate: 1.25 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 10 μL of the test solution and reference solutions (b) and (c).
Run time: 3.5 times the retention time of folinic acid.
Identification of impurities: Use the chromatogram supplied with calcium folinate for system suitability A CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A, B, C, D, E, F and I.
Relative retention: With reference to folinic acid (retention time = about 12.0 min): impurity E = about 0.4; impurity A = about 0.6; impurity F = about 0.7; impurity B = 0.8; impurity I = about 1.3 (may be eluted as 1 or 2 peaks); impurity D = about 2.1; impurity C = about 2.6.
System suitability Reference solution (c):
— resolution: minimum 2.0 between the peaks due to impurities A and F.
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 0.6; impurity B = 0.5; impurity C = 0.6; impurity D = 0.3; impurity E = 0.6; impurity F = 0.6; impurity I = 0.6;
— for each impurity, use the concentration of calcium folinate hydrate in reference solution (b).
Limits:
— impurities A, E, F: for each impurity, maximum 0.3 per cent;
— impurities B, C, D: for each impurity, maximum 0.2 per cent;
— impurity I: maximum 0.2 per cent, for the sum of the areas of the 2 peaks;
— unspecified impurities: for each impurity, maximum 0.20 per cent;
— total: maximum 1.5 per cent;
— reporting threshold: 0.05 per cent.
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph
Substances for pharmaceutical use (2034) do not apply.
Chlorides
Maximum 0.5 per cent.
Dissolve 0.300 g in 50 mL of water R heating at 40 °C if necessary. Add 10 mL of dilute nitric acid R and titrate with 0.005 M silver nitrate determining the end-point potentiometrically (2.2.20).
1 mL of 0.005 M silver nitrate is equivalent to 0.177 mg of Cl.
Water
(2.5.12): 10.0 per cent to 17.0 per cent.
Dissolve 0.100 g in a mixture of 15 mL of formamide R and 50 mL of the titration solvent. Stir for about 6 min before titrating and use a suitable titrant that does not contain pyridine.
ASSAY
Carry out the assays as rapidly as possible, protected from actinic light.
Calcium
Dissolve 0.400 g in 150 mL of water R and dilute to 300 mL with the same solvent. Carry out the complexometric titration of calcium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 4.008 mg of Ca.
Calcium folinate
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a).
Calculate the percentage content of C20H21CaN7O7 taking into account the assigned content of calcium folinate CRS.
STORAGE
In an airtight container, protected from light. If the substance is sterile, the container is also sterile and tamper-evident.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
Specified impurities A, B, C, D, E, F, I.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) G.
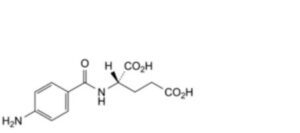
A. (2S)-2-(4-aminobenzamido)pentanedioic acid,
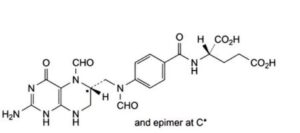
B. (2S)-2-[4-[[[(6RS)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl] (formyl)amino]benzamido]pentanedioic acid (5,10-diformyltetrahydrofolic acid),
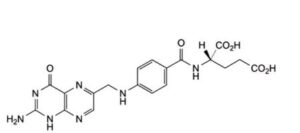
C. (2S)-2-[4-[[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino]benzamido]pentanedioic acid (folic acid),
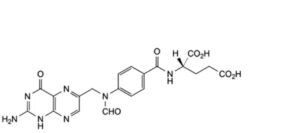
D. (2S)-2-[4-[[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl](formyl)amino]benzamido]pentanedioic acid (10-formylfolic acid),
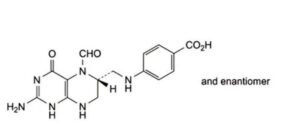
E. 4-[[[(6RS)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl]amino]benzoic acid (5-
formyltetrahydropteroic acid),
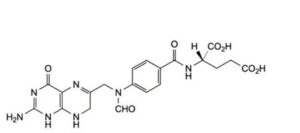
F. (2S)-2-[4-[[(2-amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl](formyl)amino]benzamido]pentanedioic acid (10-formyldihydrofolic acid),
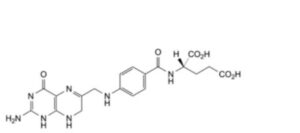
G. (2S)-2-[4-[[(2-amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]amino]benzamido]pentanedioic acid (dihydrofolic acid),
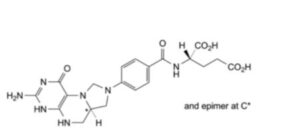
I. (2S)-2-[4-[(6aRS)-3-amino-1-oxo-1,4,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9H)-yl]benzamido]pentanedioic acid ((6aRS)-5,10-methylenetetrahydrofolic acid).






