Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 3061)
Ph Eur
DEFINITION
Sterile concentrate for solution for infusion of Cabazitaxel acetone (3060), for human use.
It complies with the monograph Parenteral preparations (0520) and the following additional requirements.
Content
95.0 per cent to 105.0 per cent of the content of cabazitaxel (C45H57NO14) stated on the label.
PRODUCTION
Manufacturers are expected to evaluate whether the presence of the active substance as a solvate is critical to the quality,
efficacy and/or safety of the medicinal product and, where applicable, implement a control strategy for the corresponding
solvent in the medicinal product, to the satisfaction of the competent authorities.
IDENTIFICATION
Carry out either tests A, B or tests B, C.
A. Record the UV spectrum of the principal peak in the chromatograms obtained with the solutions used in the assay, with a diode array detector in the range of 190-400 nm.
Results The UV spectrum of the principal peak in the chromatogram obtained with the test solution is similar to the
UV spectrum of the principal peak in the chromatogram obtained with reference solution (b).
B. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (b).
C. Thin-layer chromatography (2.2.27).
Test solution Dilute a suitable volume of the preparation to be examined with methylene chloride R to obtain a concentration of cabazitaxel of 0.4 mg/mL.
Reference solution Dissolve 4 mg of cabazitaxel acetone CRS in 10 mL of a mixture of polysorbate 80 R and methylene chloride R (1:99 V/V).
Plate TLC silica gel F254 plate R.
Mobile phase methanol R, methylene chloride R (8:92 V/V).
Cabazitaxel acetone concentrate for infusion
Application 10 μL.
Development Over 1/2 of the plate.
Detection Examine in ultraviolet light at 254 nm.
Results The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
TESTS
pH (2.2.3)
3.0 to 4.0.
Prepare a 10 per cent m/m solution of the preparation to be examined in carbon dioxide-free water R.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture acetonitrile R, methanol R, water R previously adjusted to pH 4.0 with dilute phosphoric acid R1 (26:32:42 V/V/V).
Test solution To a quantity of the preparation to be examined containing the equivalent of 20.0 mg of cabazitaxel, add 5 mL of anhydrous ethanol R and dilute to 100.0 mL with the solvent mixture.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 2.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b) Dissolve 20.0 mg of cabazitaxel acetone CRS in 5 mL of anhydrous ethanol R and dilute to 100.0 mL with the solvent mixture.
Reference solution (c) Dissolve 4 mg of cabazitaxel for peak identification CRS (containing impurities C, D, E, F and G) in 1 mL anhydrous ethanol R and dilute to 20 mL with the solvent mixture.
Reference solution (d) Dissolve 4 mg of cabazitaxel for system suitability CRS (containing impurity A) in 1 mL anhydrous ethanol R and dilute to 20 mL with the solvent mixture.
Reference solution (e) To 4 mg of cabazitaxel impurity FP-A CRS, add 5 mL of anhydrous ethanol R and 20 mL of the solvent mixture. Dissolve using sonication. Dilute 1 mL of the solution to 25 mL with the solvent mixture. Dilute 1 mL of this solution to 10 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: methanol R1, acetonitrile for chromatography R, water for chromatography R previously adjusted to pH 4.0 with dilute phosphoric acid R1 (25:35:40 V/V/V);
— mobile phase B: methanol R1, water for chromatography R previously adjusted to pH 4.0 with dilute phosphoric acid R1, acetonitrile for chromatography R (25:25:50 V/V/V);
— mobile phase C: anhydrous ethanol R, acetonitrile for chromatography R (20:80 V/V);
|
Time
(min)
|
Mobile phase A
(per cent V/V)
|
Mobile phase B
(per cent V/V)
|
Mobile phase C
(per cent V/V)
|
| 0 – 2 | 100 | 0 | 0 |
| 2 – 22 | 100 → 0 | 0 → 100 | 0 |
| 22 – 44 | 0 | 100 | 0 |
| 44 – 44.1 | 0 | 100 → 0 | 0 → 100 |
| 44.1 – 50 | 0 | 0 | 100 |
Flow rate 0.9 mL/min.
Detection Spectrophotometer at 230 nm.
Autosampler Set at 10 °C.
Injection 25 μL of the test solution and reference solutions (a), (c), (d) and (e). Identification of impurities Use the chromatogram supplied with cabazitaxel for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities C, D + F, E and G; use the chromatogram supplied with cabazitaxel for system suitability CRS and the chromatogram obtained with reference solution (d) to identify the peak due to impurity A; use the chromatogram obtained with reference solution (e) to identify the peak due to impurity FP-A.
Relative retention With reference to cabazitaxel (retention time = about 19 min): impurity FP-A = about 0.4;
impurity A = about 0.96; impurity C = about 1.4; impurities D + F = about 1.61; impurity E = about 1.65;
impurity G = about 1.74.
System suitability Reference solution (d):
— resolution : minimum 1.5 between the peaks due to impurity A and cabazitaxel.
Calculation of percentage contents:
— correction factor: multiply the peak area of impurity FP-A by 0.7;
— for each impurity, use the concentration of cabazitaxel in reference solution (a).
Limits:
— impurity FP-A: maximum 0.30 per cent;
— unspecified impurities: for each impurity, maximum 0.20 per cent;
— total: maximum 0.80 per cent;
— reporting threshold: 0.10 per cent; disregard the peaks due to impurities A, C, D + F, E and G.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection Test solution and reference solution (b).
System suitability Reference solution (b):
— repeatability: maximum relative standard deviation of 1.0 per cent determined on 6 injections.
Calculate the percentage content of cabazitaxel (C45H57NO14), taking into account the assigned content of cabazitaxel acetone CRS.
IMPURITIES
Specified impurities FP-A.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph): A, B, C, D, E, F, G, H.
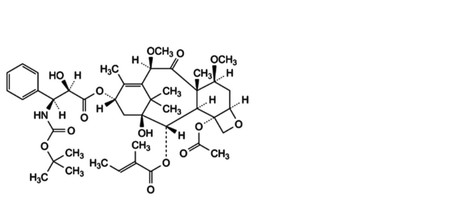
A. 4-(acetyloxy)-13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl (2E)-2-methylbut-2-enoate,
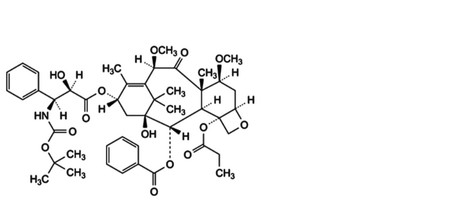
B. 13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β,10β-dimethoxy-9-oxo-4-(propanoyloxy)-5β,20-epoxytax-11-en-2α-yl benzoate,
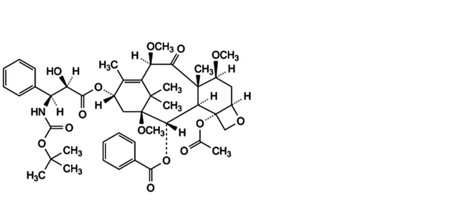
C. 4-(acetyloxy)-13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1,7β,10β-trimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
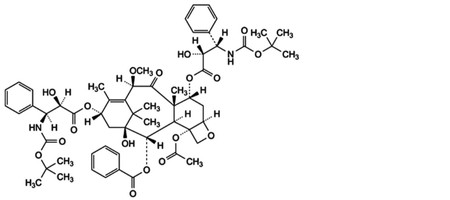
D. 4-(acetyloxy)-7α,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-10β-methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
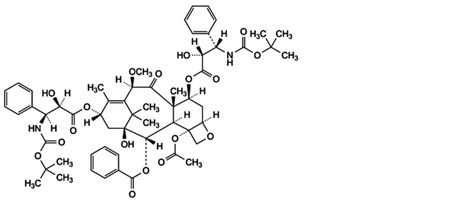
E. 4-(acetyloxy)-7β,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-10β-methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
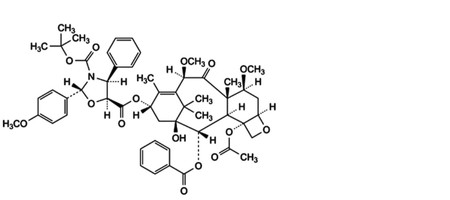
F. 5-[4-(acetyloxy)-2α-(benzoyloxy)-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-13α-yl] 3-tert-butyl(2R,4S,5S)-2-(4-methoxyphenyl)-4-phenyl-1,3-oxazolidine-3,5-dicarboxylate,
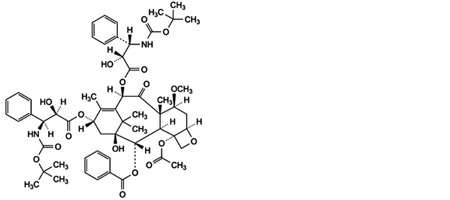
G. 4-(acetyloxy)-10β,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β-methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
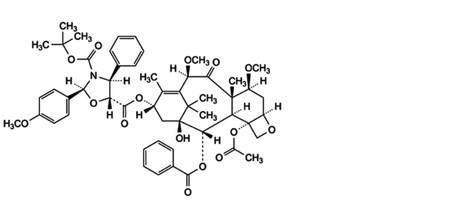
H. 5-[4-(acetyloxy)-2α-(benzoyloxy)-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-13α-yl] 3-tert-butyl(2S,4S,5R)-2-(4-methoxyphenyl)-4-phenyl-1,3-oxazolidine-3,5-dicarboxylate,
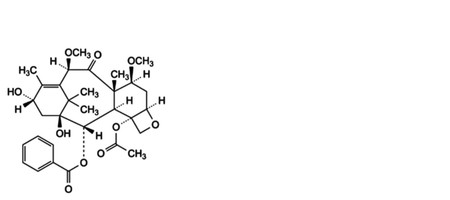
FP-A. 4-(acetyloxy)-1,13α-dihydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate.






