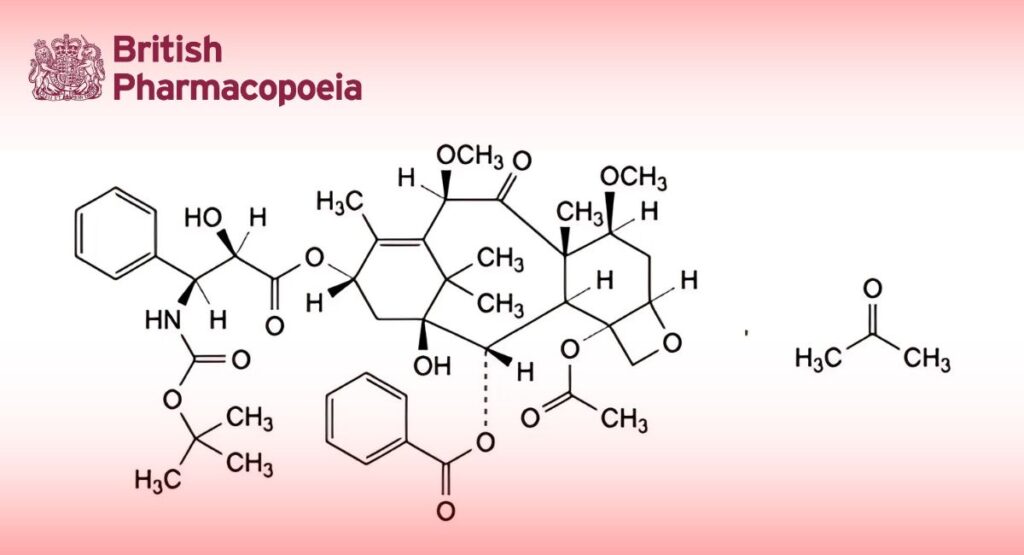
(Ph. Eur. monograph 3060)
C45H57NO14,C3H6O 894 1426815-65-7
Action and use
Taxane antineoplastic; treatment of prostate carcinoma.
Preparation
Cabazitaxel Concentrate for Infusion
DEFINITION
4-(Acetyloxy)-13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β,10β- dimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate as a solvate with propan-2-one.
Content
97.0 per cent to 102.0 per cent (anhydrous and acetone free substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Practically insoluble in water, soluble in anhydrous ethanol, practically insoluble in heptane.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: cabazitaxel acetone CRS.
B. Acetone (see Tests).
TESTS
Specific optical rotation (2.2.7)
-44 to -40 (anhydrous and acetone free substance).
Dissolve 0.125 g in methanol R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture: acetonitrile R, water R previously adjusted to pH 4.0 with dilute phosphoric acid R1 (35:65 V/V).
Test solution: Dissolve 50.0 mg of the substance to be examined in 20 mL of acetonitrile R and dilute to 50.0 mL with the solvent mixture. Dilute 5.0 mL of the solution to 20.0 mL with the solvent mixture.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b): Dissolve 50.0 mg of cabazitaxel acetone CRS in 20 mL of acetonitrile R and dilute to 50.0 mL with the solvent mixture. Dilute 5.0 mL of the solution to 20.0 mL with the solvent mixture.
Reference solution (c): Dissolve 5 mg of cabazitaxel for peak identification CRS (containing impurities C, D, E, F and G) in 2 mL of acetonitrile R and dilute to 20 mL with the solvent mixture.
Reference solution (d): Dissolve 5 mg of cabazitaxel for system suitability CRS (containing impurity A) in 2 mL of acetonitrile R and dilute to 20 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 3.0 mm;
— stationary phase: end-capped solid core octadecylsilyl silica gel for chromatography R (2.7 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: acetonitrile for chromatography R, water for chromatography R (35:65 V/V);
— mobile phase B: water for chromatography R, acetonitrile for chromatography R (25:75 V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 100 | 0 |
| 2 – 9 | 100 → 62 | 0 → 38 |
| 9 – 38 | 62 → 22 | 38 → 78 |
Flow rate: 0.6 mL/min.
Detection: Spectrophotometer at 230 nm.
Autosampler: Set at 10 °C.
Injection: 10 μL.
Identification of impurities: Use the chromatogram supplied with cabazitaxel for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities C, D + E, F and G; use the chromatogram supplied with cabazitaxel for system suitability CRS and the chromatogram obtained with reference solution (d) to identify the peak due to impurity A.
Relative retention: With reference to cabazitaxel (retention time = about 16 min): impurity A = about 0.9; impurity C = about 1.4; impurities D + E = about 1.8; impurity F = about 1.9; impurity G = about 1.95.
System suitability: Reference solution (d):
— resolution: minimum 1.5 between the peaks due to cabazitaxel and impurity A.
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 1.3; impurities D + E = 1.4; impurity F = 0.7;
— for each impurity, use the concentration of cabazitaxel acetone in reference solution (a).
Limits:
— impurities A, G: for each impurity, maximum 0.2 per cent;
— sum of impurities D and E: maximum 0.2 per cent;
— impurities C, F: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.8 per cent;
— reporting threshold: 0.05 per cent.
Acetone
Head-space gas chromatography (2.2.28).
Internal standard solution: Mix 1.0 mL of methyl isobutyl ketone R and 10 mL of dimethyl sulfoxide R, and dilute to 100.0 mL with dimethyl sulfoxide R.
Test solution: Dissolve 0.100 g of the substance to be examined in 1.0 mL of the internal standard solution and dilute to 10.0 mL with dimethyl sulfoxide R.
Reference solution: Mix 0.650 g of acetone R and 10 mL of dimethyl sulfoxide R, and dilute to 20.0 mL with dimethyl sulfoxide R. To 1.0 mL of this solution add 5.0 mL of internal standard solution and dilute to 50.0 mL with dimethyl sulfoxide R.
Close the vials immediately with a butyl rubber membrane stopper coated with polytetrafluoroethylene and secured with an aluminium crimp cap. Mix to obtain a homogeneous solution.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.32 mm;
— stationary phase: trifluoropropylmethylpolysiloxane R (film thickness 1 μm).
Carrier gas: helium for chromatography R.
Flow rate: 2.5 mL/min.
Split ratio: 1:20.
Static head-space conditions that may be used:
— equilibration temperature: 110 °C;
— equilibration time: 15 min;
— injection time: 1 min;
— injection volume: 1.0 mL.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 6 | 35 |
| 6 – 11.5 | 35 → 120 | |
| 11.5 – 15.5 | 120 → 220 | |
| 15.5 – 25.5 | 220 | |
| Injection port | 200 | |
| Detector | 250 |
Detection: FLame ionisation.
Identification of peaks: Use the chromatogram obtained with the reference solution to identify the peak due to acetone.
Relative retention: With reference to methyl isobutyl ketone (retention time = about 9.7 min): acetone = about 0.4.
System suitability: Reference solution:
— repeatability: maximum relative standard deviation of 3.0 per cent determined on 3 injections.
Calculation of percentage content: Use the internal standard method.
Limit:
— acetone: 5.0 per cent m/m to 7.0 per cent m/m.
Water (2.5.32)
Maximum 1.0 per cent, determined on 200 mg by direct sample introduction. [Stir for a minimum of 180 s.]
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
Bacterial endotoxins (2.6.14, Method D)
Solution A: A 0.20 mg/mL solution of the substance to be examined in a 0.25 per cent V/V solution of polysorbate 80 R.
Solution C: A 0.5 per cent V/V solution of polysorbate 80 R.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution and reference solution (b).
Calculate the percentage content of C45H57NO14 taking into account the assigned content of cabazitaxel acetone CRS.
STORAGE
At a temperature of 2 °C to 8 °C.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
Specified impurities A, C, D, E, F, G.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, H.
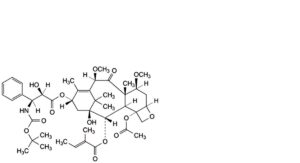
A. 4-(acetyloxy)-13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl (2E)-2-methylbut-2-enoate,
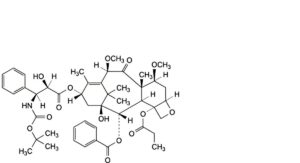
B. 13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β,10β-dimethoxy-9-oxo-4-(propanoyloxy)-5β,20-epoxytax-11-en-2α-yl benzoate,
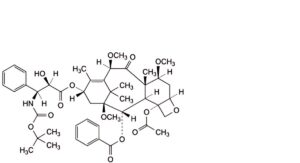
C. 4-(acetyloxy)-13α-[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1,7β,10β-trimethoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
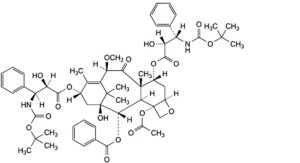
D. 4-(acetyloxy)-7α,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-10β-methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
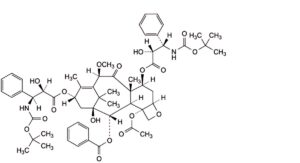
E. 4-(acetyloxy)-7β,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-10β-methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
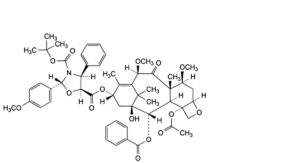
F. 5-[4-(acetyloxy)-2α-(benzoyloxy)-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-13α-yl] 3-tert-butyl (2R,4S,5S)-2-(4-methoxyphenyl)-4-phenyl-1,3-oxazolidine-3,5-dicarboxylate,
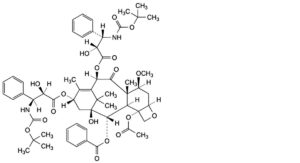
G. 4-(acetyloxy)-10β,13α-bis[[(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-7β- methoxy-9-oxo-5β,20-epoxytax-11-en-2α-yl benzoate,
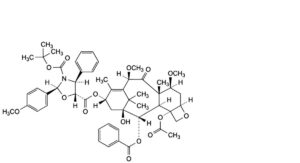
H. 5-[4-(acetyloxy)-2α-(benzoyloxy)-1-hydroxy-7β,10β-dimethoxy-9-oxo-5β,20-epoxytax-11-en-13α-yl] 3-tert-butyl (2S,4S,5R)-2-(4-methoxyphenyl)-4-phenyl-1,3-oxazolidine-3,5-dicarboxylate.