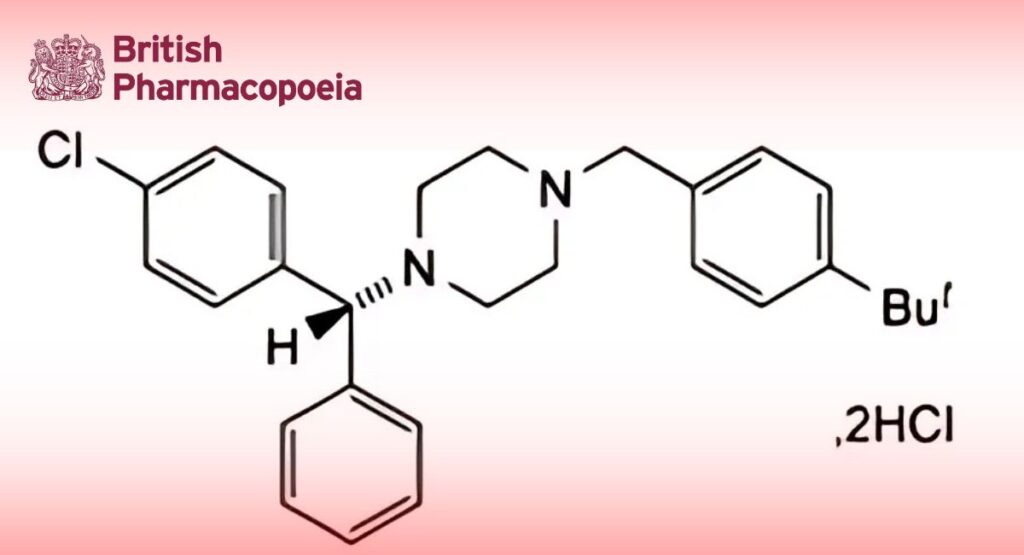
C28H33ClN2,2HCl 506.0 129-74-8
Action and use
Histamine H1 receptor antagonist; antiemetic.
DEFINITION
Buclizine Hydrochloride is (RS)-1-(4-tert-butylbenzyl)-4-(4-chlorobenzhydryl)piperazine dihydrochloride. It contains not less than 99.0% and not more than 100.5% of C28H33ClN2,2HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A white or slightly yellowish, crystalline powder.
Practically insoluble in water; sparingly soluble in propane-1,2-diol; very slightly soluble in ethanol (96%).
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of buclizine hydrochloride (RS 032).
B. A 0.25% w/v solution in ethanol (50%) yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using four solutions in the initial mobile phase containing (1) 0.0010% w/v of the substance being examined, (2) 0.50% w/v of the substance being examined, (3) 0.0010% w/v of 1,4-bis(4-chlorobenzhydryl)piperazine BPCRS and (4) 0.50% w/v of buclizine hydrochloride impurity standard BPCRS.
The chromatographic procedure may be carried out using a stainless steel column (20 cm × 4 mm) packed with octadecylsilyl silica gel for chromatography (10 μm) (Nucleosil C18 is suitable). Use as the initial mobile phase 0.01M sodium heptanesulfonate in a mixture of 55 volumes of water and 45 volumes of acetonitrile and as the final mobile phase 0.01M sodium heptanesulfonate in a mixture of 20 volumes of water and 80 volumes of acetonitrile. Before use, adjust the pH of both the initial and final mobile phases to 4.0 with 1M orthophosphoric acid. Carry out a linear gradient elution with a flow rate of 2 mL per minute for 30 minutes and maintain the final mobile phase for 10 minutes with the same flow rate. Use a detection wavelength of 230 nm.
The test is not valid unless the chromatogram obtained with solution (4) closely resembles the chromatogram supplied with buclizine hydrochloride impurity standard BPCRS.
In the chromatogram obtained with solution (2) the area of any peak corresponding to 1,4-bis(4-chlorobenzhydryl)- piperazine is not greater than the area of the peak obtained in the chromatogram with solution (3) and the area of any other secondary peak is not greater than the area of the peak in the chromatogram obtained with solution (1).
Loss on drying
When dried to constant weight at 100° to 105°, loses not more than 1.0% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.4 g and determining the end point potentiometrically.
Each mL of 0.1M perchloric acid VS is equivalent to 25.30 mg of C28H33ClN2,2HCl.
IMPURITIES
A. 1,4-bis(4-chlorobenzhydryl)piperazine,
B. 4-chlorobenzhydrol, 1-(4-chlorobenzhydryl)piperazine, 4-chlorobenzophenone.