Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 3142)
Action and use
Anticonvulsant; synaptic vesicle protein 2A (SV2A) ligand; adjunctive therapy of partial-onset seizures.
Ph Eur
DEFINITION
Sterile solution for injection or infusion of Brivaracetam (3139), for human use.
It complies with the monograph Parenteral preparations (0520) and the following additional requirements.
Content
95.0 per cent to 105.0 per cent of the content of brivaracetam (C11H20N2O2) stated on the label.
IDENTIFICATION
A. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (f).
B. Thin-layer chromatography (2.2.27).
Test solution The preparation to be examined.
Reference solution Dissolve 10 mg of brivaracetam CRS in 1 mL of methylene chloride R.
Plate TLC silica gel F254 plate R (5-40 μm) [or TLC silica gel F254 plate R (2-10 μm)].
Mobile phase concentrated ammonia R, methanol R, ethyl acetate R (3:12:85 V/V/V).
Application 20 μL, as bands of 10 mm.
Development Over 1/2 of the plate.
Drying In air for 1-2 min.
Detection Expose to iodine vapour until the spots appear and examine in daylight.
Results The spot in the chromatogram obtained with the test solution is similar in position to the spot in the chromatogram obtained with the reference solution.
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution Dilute a suitable volume of the preparation to be examined with water R to obtain a concentration of brivaracetam of 0.50 mg/mL.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with water R. Dilute 2.0 mL of this solution to 10.0 mL with water R.
Reference solution (b) Dissolve 3 mg of brivaracetam R and 3 mg of brivaracetam impurity A CRS in water R and dilute to 5 mL with the same solvent.
Reference solution (c) Dissolve 5 mg of brivaracetam impurity D CRS and 5 mg of brivaracetam impurity E CRS in water R and dilute to 100 mL with the same solvent. Dilute 1 mL of this solution to 50 mL with water R.
Reference solution (d) Dissolve 65.0 mg of brivaracetam CRS in water R and dilute to 100.0 mL with the same solvent.
Reference solutions (e), (f), (g), (h) Dilute reference solution (d) with water R as necessary to obtain reference solutions with a concentration of 0.58 mg/mL, 0.50 mg/mL (reference solution (f)), 0.43 mg/mL and 0.35 mg/mL.
A precolumn containing end-capped ethylene-bridged octadecylsilyl silica gel for chromatography (hybrid material) R (1.7 μm) may be used.
Column:
— size: l = 0.10 m, Ø = 2.1 mm;
— temperature: 38 °C;
— stationary phase: end-capped ethylene-bridged octadecylsilyl silica gel for chromatography (hybrid material) R
(1.7 μm).
Mobile phase:
— mobile phase A: formic acid R, water for chromatography R (0.1:1000 V/V);
— mobile phase B: formic acid R, acetonitrile R1 (0.1:1000 V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 1 | 92 | 8 |
| 1 – 12 | 92 → 72 | 8 → 28 |
| 12 – 12.5 | 72 → 50 | 28 →50 |
| 12.5 – 13.5 | 50 | 50 |
Flow rate 0.5 mL/min.
Detection Spectrophotometer at 205 nm.
Injection 3 μL of the test solution and reference solutions (a), (b) and (c).
Identification of impurities Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity A; use the chromatogram obtained with reference solution (c) to identify the peaks due to impurities D and E.
Relative retention With reference to brivaracetam (retention time = about 8 min): impurity A = about 1.04;
impurity D = about 1.2; impurity E = about 1.3.
System suitability Reference solution (b):
— resolution: minimum 2.0 between the peaks due to brivaracetam and impurity A.
Calculation of percentage contents:
— for each impurity, use the concentration of brivaracetam in reference solution (a).
Limits:
— sum of impurities D and E: maximum 0.50 per cent;
— unspecified impurities: for each impurity, maximum 0.20 per cent;
— total: maximum 0.80 per cent;
— reporting threshold: 0.10 per cent; disregard the peak due to impurity A.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances, with the following modifications.
Injection Test solution and reference solutions (d), (e), (f), (g) and (h).
System suitability:
— repeatability: maximum relative standard deviation of 1.0 per cent determined on 6 injections of reference solution (f);
— the coefficient of determination (r ) calculated for the calibration curve is not less than 0.995.
Calculate the percentage content of C11H20N2O2 using the calibration curve and taking into account the assigned content of brivaracetam CRS.
IMPURITIES
Specified impurities D, E.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph): A.
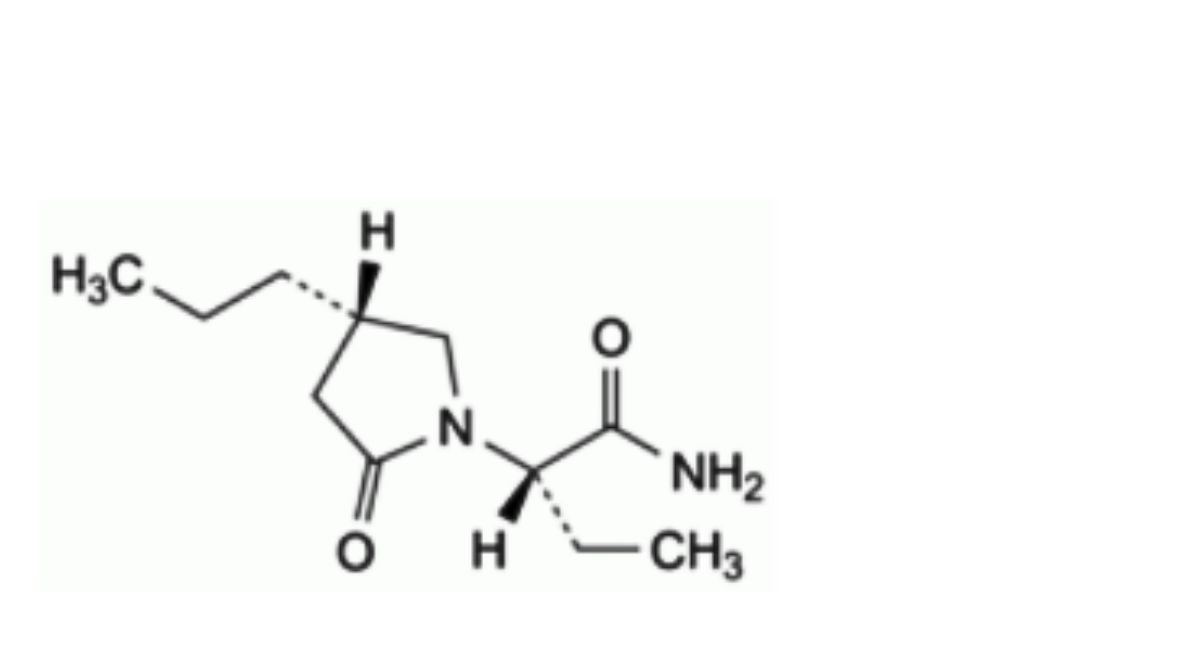
A. (2S)-2-[(4S)-2-oxo-4-propylpyrrolidin-1-yl]butanamide,
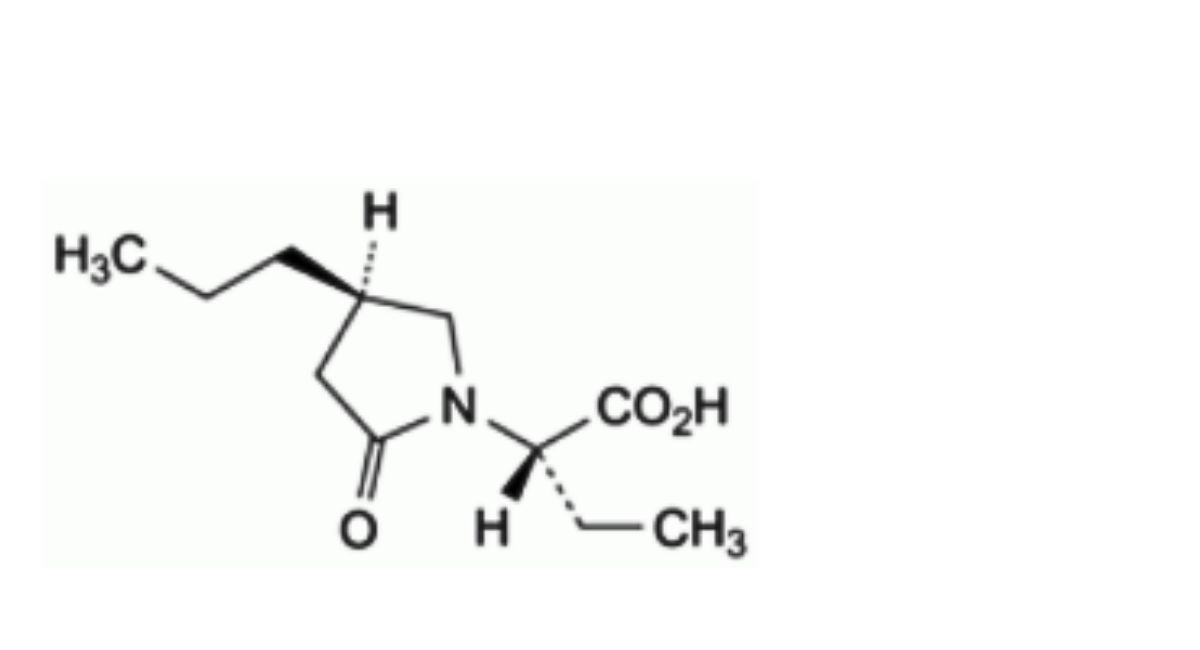 D. (2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]butanoic acid,
D. (2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]butanoic acid,
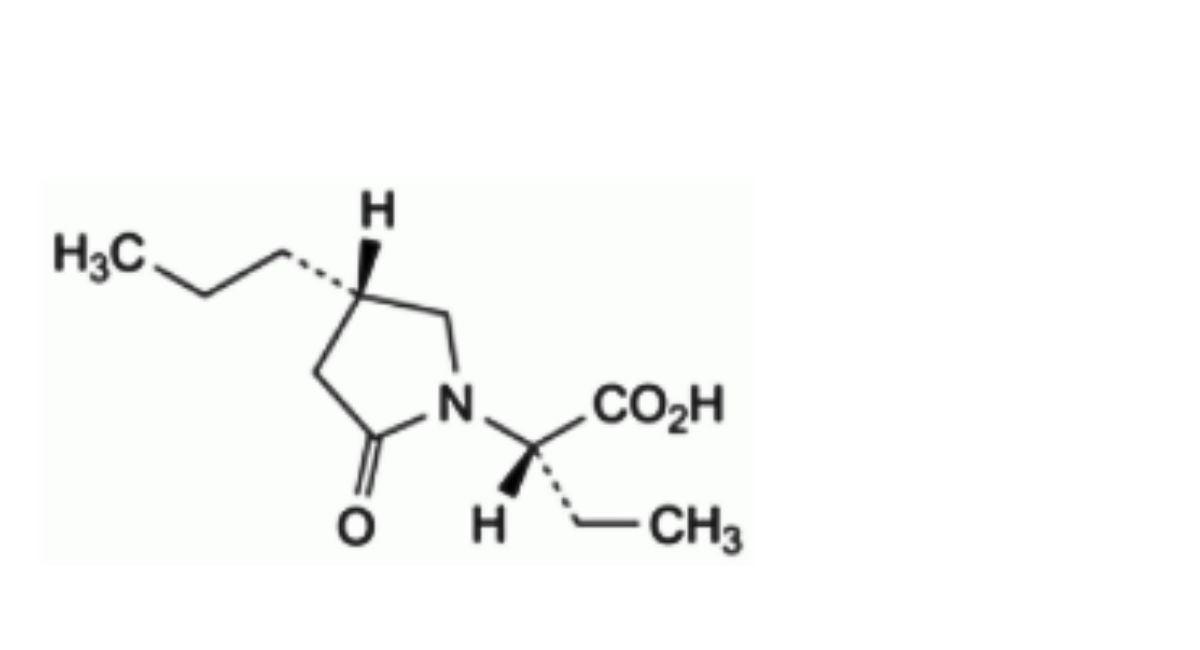
E. (2S)-2-[(4S)-2-oxo-4-propylpyrrolidin-1-yl]butanoic acid.
Ph Eur






