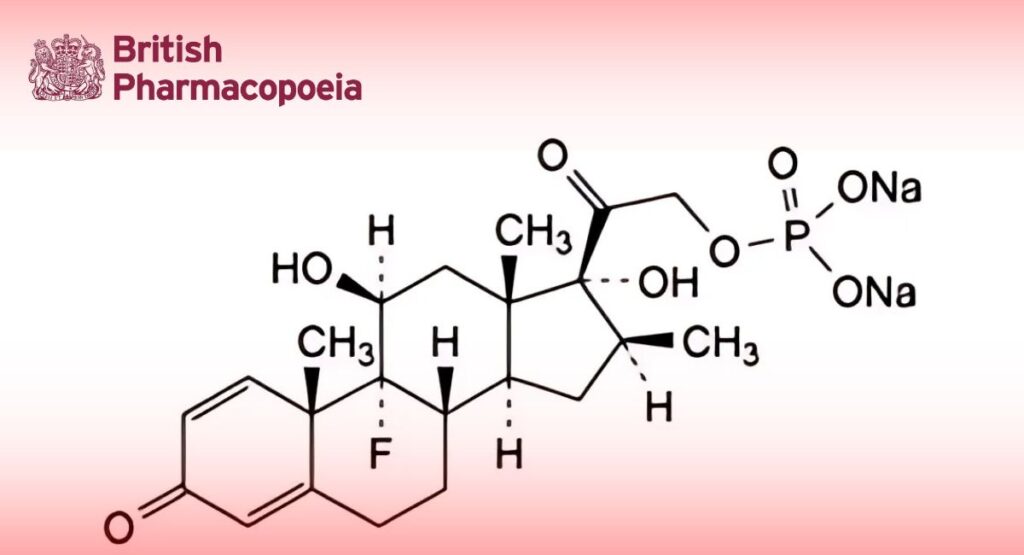
(Ph. Eur. monograph 0810)
C22H28FNa2O8P 516.4 151-73-5
Action and use
Glucocorticoid.
Preparations
Betamethasone Eye Drops
Betamethasone Injection
Betamethasone Sodium Phosphate Tablets
DEFINITION
Disodium 9-fluoro-11β,17-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-dien-21-yl phosphate.
Content
96.0 per cent to 103.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, very hygroscopic powder.
Solubility
Freely soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: A, B.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison betamethasone sodium phosphate CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in the minimum volume of ethanol (96 per cent) R, evaporate to dryness on a water-bath and record new spectra using the residues.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methanol R and dilute to 10.0 mL with the same solvent.
Reference solution: Dissolve 10 mg of betamethasone sodium phosphate CRS in methanol R and dilute to 10.0 mL with the same solvent.
Plate: TLC silica gel F254 plate R.
Mobile phase: glacial acetic acid R, water R, butanol R (20:20:60 V/V/V).
Application: 5 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection: Spray with a solution prepared as follows: dissolve 0.25 g of 2,4-dihydroxybenzaldehyde R in glacial acetic acid R, dilute to 50 mL with the same solvent and add a mixture of 12.5 mL of sulfuric acid R and 37.5 mL of glacial acetic acid R; heat the plate at 90 °C for 35 min or until the spots appear and allow to cool; examine in daylight and in ultraviolet light at 365 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Add about 2 mg to 2 mL of sulfuric acid R and shake to dissolve. Within 5 min, an intense reddish-brown colour develops. Add the solution to 10 mL of water R and mix. The colour disappears and a clear solution remains.
TESTS
Solution S
Dissolve 1.0 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B7 (2.2.2, Method II).
pH (2.2.3)
7.5 to 9.0.
Dilute 1 mL of solution S to 5 mL with carbon dioxide-free water R.
Specific optical rotation (2.2.7)
+ 98 to + 104 (anhydrous substance).
Dissolve 0.250 g in water R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 62.5 mg of the substance to be examined in the mobile phase and dilute to 25.0 mL with the mobile phase.
Reference solution (a): Dissolve 25 mg of betamethasone sodium phosphate CRS and 25 mg of dexamethasone sodium phosphate CRS in the mobile phase and dilute to 25 mL with the mobile phase. Dilute 1 mL of this solution to 25 mL with the mobile phase.
Reference solution (b): Dilute 1.0 mL of the test solution to 50.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: In a 250 mL conical flask, weigh 1.360 g of potassium dihydrogen phosphate R and 0.600 g of hexylamine R, mix and allow to stand for 10 min and then dissolve in 185 mL of water for chromatography R; add 65 mL of acetonitrile R, mix and filter (0.45 μm).
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 254 nm.
Equilibration: With the mobile phase for about 45 min.
Injection: 20 μL.
Run time: Twice the retention time of betamethasone sodium phosphate.
Retention time: Betamethasone sodium phosphate = about 14 min; dexamethasone sodium phosphate = about 15.5 min.
System suitability: Reference solution (a):
— resolution: minimum 2.0 between the peaks due to betamethasone sodium phosphate and dexamethasone sodium phosphate; if necessary, increase the concentration of acetonitrile R or increase the concentration of water for chromatography R in the mobile phase.
Limits:
— any impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2 per cent), and not more than 1 such peak has an area greater than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1 per cent);
— total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3 per cent);
— disregard limit: 0.025 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Inorganic phosphate
Maximum 1 per cent.
Dissolve 50 mg in water R and dilute to 100 mL with the same solvent. To 10 mL of the solution add 5 mL of molybdovanadic reagent R, mix and allow to stand for 5 min. Any yellow colour in the solution is not more intense than that in a standard prepared at the same time and in the same manner using 10 mL of phosphate standard solution (5 ppm PO4) R.
Water (2.5.12)
Maximum 8.0 per cent, determined on 0.200 g.
ASSAY
Dissolve 0.100 g in water R and dilute to 100.0 mL with the same solvent. Dilute 5.0 mL of the solution to 250.0 mL with water R. Measure the absorbance (2.2.25) at the absorption maximum at 241 nm.
Calculate the content of C22H28FNa2O8P taking the specific absorbance to be 297.
STORAGE
In an airtight container, protected from light.