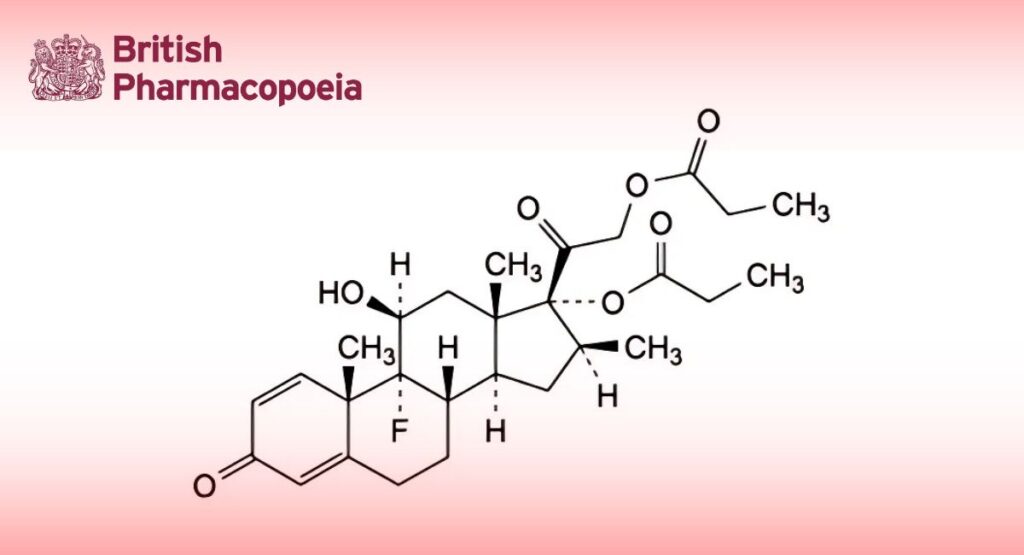
(Ph. Eur. monograph 0809)
C28H37FO7 504.6 5593-20-4
Action and use
Glucocorticoid.
Preparations
Calcipotriol and Betamethasone Cutaneous Foam
Calcipotriol and Betamethasone Gel
Calcipotriol and Betamethasone Ointment
DEFINITION
9-Fluoro-11β-hydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate.
Content
97.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification:
A.Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison betamethasone dipropionate CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution: Dissolve 10 mg of betamethasone dipropionate CRS in the mobile phase and dilute to 10.0 mL with the mobile phase.
Plate: TLC silica gel F254 plate R.
Mobile phase methanol R, methylene chloride R (10:90 V/V).
Application 5 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection: Spray with a solution prepared as follows: dissolve 0.25 g of 2,4-dihydroxybenzaldehyde R in glacial acetic acid R, dilute to 50 mL with the same solvent and add a mixture of 12.5 mL of sulfuric acid R and 37.5 mL of glacial acetic acid R; heat the plate at 90 °C for 35 min or until the spots appear, allow to cool and examine in daylight and in ultraviolet light at 365 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Add about 2 mg to 2 mL of sulfuric acid R and shake to dissolve. Within 5 min, a deep reddish-brown colour develops. Add this solution to 10 mL of water R and mix. The colour is discharged and a clear solution remains.
TESTS
Specific optical rotation (2.2.7)
+ 84 to + 88 (dried substance).
Dissolve 0.250 g in anhydrous ethanol R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 60.0 mg of the substance to be examined in the mobile phase and dilute to 25.0 mL with the mobile phase.
Test solution (b): Dilute 1.0 mL of test solution (a) to 10.0 mL with the mobile phase.
Reference solution (a): Dissolve 5 mg of betamethasone dipropionate for system suitability A CRS (containing impurities B, C, D, E, G and I) in the mobile phase and dilute to 2 mL with the mobile phase.
Reference solution (b): Dilute 1.0 mL of test solution (a) to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (c): Dissolve 60.0 mg of betamethasone dipropionate CRS in the mobile phase and dilute to 25.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 10.0 mL with the mobile phase.
Reference solution (d): Dissolve 5 mg of betamethasone dipropionate for peak identification CRS (containing impurity H) in the mobile phase and dilute to 2 mL with the mobile phase.
Column:
— size: l = 0.10 m, Ø = 2.0 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (2.5 μm);
— temperature: 20 ± 2 °C.
Mobile phase: Mix 35 mL of water for chromatography R and 56 mL of acetonitrile R and allow to equilibrate; dilute to 100 mL with water for chromatography R and mix.
Flow rate: 0.2 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 5 μL of test solution (a) and reference solutions (a), (b) and (d).
Run time: 3 times the retention time of betamethasone dipropionate.
Identification of impurities: Use the chromatogram supplied with betamethasone dipropionate for system suitability A CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities B, C, D, E, G and I; use the chromatogram supplied with betamethasone dipropionate for peak identification CRS and the chromatogram obtained with reference solution (d) to identify the peak due to impurity H.
Relative retention: With reference to betamethasone dipropionate (retention time = about 10 min): impurity B = about 0.4; impurity C = about 0.5; impurity D = about 0.7; impurity I = about 1.16; impurity E = about 1.22; impurity H = about 1.7; impurity G = about 2.1.
System suitability: Reference solution (a):
— peak-to-valley ratio: minimum 2.0, where Hp = height above the baseline of the peak due to impurity I and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to betamethasone dipropionate; minimum 4.0, where Hp = height above the baseline of the peak due to impurity I and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity E.
Limits:
— correction factors: for the calculation of content, multiply the peak areas of the following impurities by the corresponding correction factor: impurity G = 1.3; impurity H = 1.4;
— impurity C: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— impurities B, H: for each impurity, not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— impurities D, E, G: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— impurity I: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 10 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 0.500 g by drying in an oven at 105 °C.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution (b) and reference solution (c).
Calculate the percentage content of C28H37FO7 taking into account the assigned content of betamethasone dipropionate CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities B, C, D, E, G, H, I.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph
Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, F.
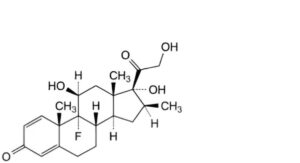
A. 9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione (betamethasone),
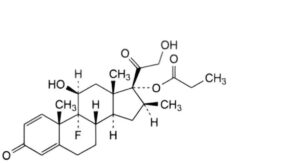
B. 9-fluoro-11β,21-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-dien-17-yl propanoate (betamethasone 17-propionate),
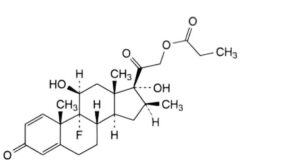
C. 9-fluoro-11β,17-dihydroxy-16β-methyl-3,20-dioxopregna-1,4-dien-21-yl propanoate (betamethasone 21-propionate),
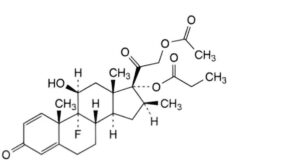
D. 9-fluoro-11β-hydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl 21-acetate 17-propanoate (betamethasone 21-acetate 17-propionate),
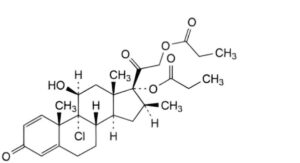
E. 9-chloro-11β-hydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate (beclometasone dipropionate),
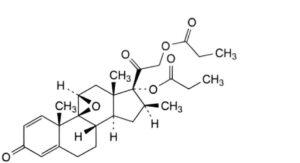
F. 9,11β-epoxy-16β-methyl-3,20-dioxo-9β-pregna-1,4-diene-17,21-diyl dipropanoate (9β,11β-epoxybetamethasone dipropionate),
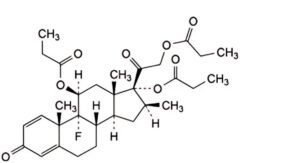
G. 9-fluoro-16β-methyl-3,20-dioxopregna-1,4-diene-11β,17,21-triyl tripropanoate (betamethasone tripropionate),
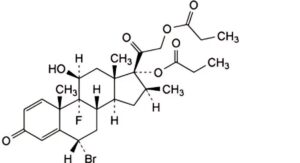
H. 6α-bromo-9-fluoro-11β-hydroxy-16β-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate (6α-bromobetamethasone dipropionate),
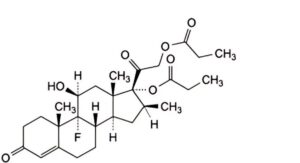
I. 9-fluoro-11β-hydroxy-16β-methyl-3,20-dioxopregn-4-ene-17,21-diyl dipropanoate (1,2-dihydrobetamethasone dipropionate).