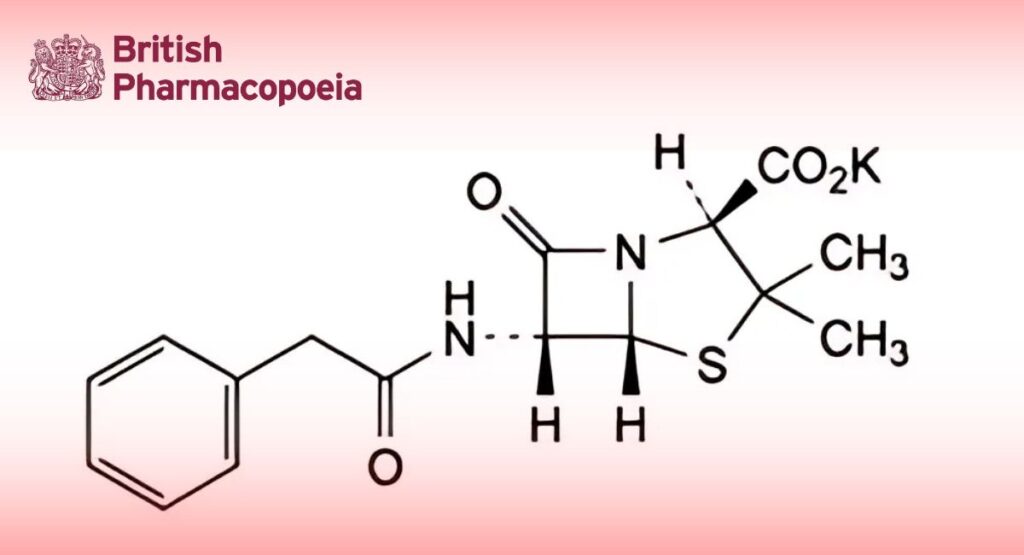
(Ph. Eur. monograph 0113)
C16H17KN2O4S 372.5 113-98-4
Action and use
Penicillin antibacterial.
Preparation
Benzylpenicillin for Injection
DEFINITION
Potassium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate.
Substance produced by the growth of certain strains of Penicillium notatum or related organisms.
Content
95.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, slightly hygroscopic, crystalline powder.
Solubility
Very soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: benzylpenicillin potassium CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 25 mg of the substance to be examined in 5 mL of water R.
Reference solution (a): Dissolve 25 mg of benzylpenicillin potassium CRS in 5 mL of water R.
Reference solution (b): Dissolve 25 mg of benzylpenicillin potassium CRS and 25 mg of phenoxymethylpenicillin potassium CRS in 5 mL of water R.
Plate: TLC silanised silica gel plate R.
Mobile phase: Mix 30 volumes of acetone R and 70 volumes of a 154 g/L solution of ammonium acetate R previously adjusted to pH 5.0 with glacial acetic acid R.
Application: 1 μL.
Development: Over 2/3 of the plate.
Drying: In air.
Detection: Expose to iodine vapour until the spots appear and examine in daylight.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Place about 2 mg in a test-tube about 150 mm long and 15 mm in diameter. Moisten with 0.05 mL of water R and add 2 mL of sulfuric acid-formaldehyde reagent R. Mix the contents of the tube by swirling; the solution is practically colourless.
Place the test-tube on a water-bath for 1 min; a reddish-brown colour develops.
D. It gives reaction (a) of potassium (2.3.1).
TESTS
pH (2.2.3)
5.5 to 7.5.
Dissolve 2.0 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
Appearance of solution
The solution is clear (2.2.1).
Dissolve 3.0 g in water R and dilute to 10 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution (a): Dissolve 50.0 mg of the substance to be examined in water R and dilute to 50.0 mL with the same solvent.
Test solution (b): Dissolve 80.0 mg of the substance to be examined in water R and dilute to 20.0 mL with the same solvent.
Reference solution (a): Dissolve 50.0 mg of benzylpenicillin sodium CRS in water R and dilute to 50.0 mL with the same solvent.
Reference solution (b): Dissolve 5 mg of benzylpenicillin for system suitability CRS (containing impurities A, B, C, D, E, F, G and H) in 0.35 mL of methanol R1 and add 0.65 mL of water R.
Reference solution (c): Dilute 1.0 mL of test solution (b) to 100.0 mL with water R.
Reference solution (d): Dilute 1.0 mL of reference solution (c) to 20.0 mL with water R.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 50 °C.
Mobile phase:
— mobile phase A: mix 10 volumes of a 68 g/L solution of potassium dihydrogen phosphate R adjusted to pH 3.4 with a 500 g/L solution of phosphoric acid R, 30 volumes of methanol R1 and 60 volumes of water for chromatography R;
— mobile phase B: mix 10 volumes of a 68 g/L solution of potassium dihydrogen phosphate R adjusted to pH 3.4 with a 500 g/L solution of phosphoric acid R, 35 volumes of water for chromatography R and 55 volumes of methanol R1;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 7 | 70 | 30 |
| 7 – 17 | 70 → 0 | 30 → 100 |
| 17 – 22 | 0 | 100 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 225 nm.
Injection: 20 μL of test solution (b) and reference solutions (b), (c) and (d).
Identification of impurities: Use the chromatogram supplied with benzylpenicillin for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B, C, D, E, F, G and H.
Relative retention: With reference to benzylpenicillin (retention time = about 7 min): impurity A = about 0.22; impurity D = about 0.33; impurity C = about 0.35; impurity E = about 0.48 and 0.55; impurity B = about 0.62; impurity F = about 0.81 and 0.83; impurity G = about 1.47; impurity H = about 1.90.
System suitability:
— resolution: minimum 1.2 between the peaks due to the epimers of impurity F and minimum 1.5 between the peaks due to impurities D and C in the chromatogram obtained with reference solution (b);
— signal-to-noise ratio: minimum 20 for the principal peak in the chromatogram obtained with reference solution (d).
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 1.4; impurity D = 0.6; impurity E = 2.0; impurity F = 1.7;
— for each impurity, use the concentration of benzylpenicillin potassium in reference solution (c).
Limits: — impurity F: maximum 2.0 per cent for the sum of the 2 epimers;
— impurity E: maximum 1.0 per cent for the sum of the isomers;
— impurity B: maximum 0.5 per cent;
— impurities A, C, D, G, H: for each impurity, maximum 0.2 per cent;
— any other impurity: for each impurity, maximum 0.2 per cent;
— total: maximum 3.0 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase: Mobile phase A, mobile phase B (70:30 V/V).
Flow rate: 1.2 mL/min.
Injection: 10 μL of test solution (a) and reference solution (a).
Calculate the percentage content of C16H17KN2O4S taking into account the assigned content of benzylpenicillin sodium CRS and a conversion factor of 1.045.
STORAGE
In an airtight container. If the substance is sterile, the container is also sterile and tamper-evident.
IMPURITIES
Specified impurities A, B, C, D, E, F, G, H.
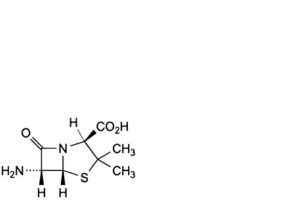
A. (2S,5R,6R)-6-amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (6-aminopenicillanic acid),
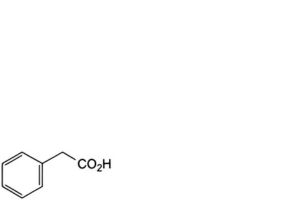
B. phenylacetic acid,
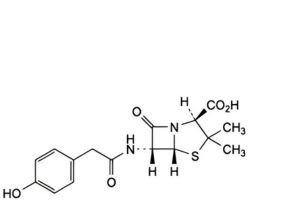
C. (2S,5R,6R)-6-[[(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,
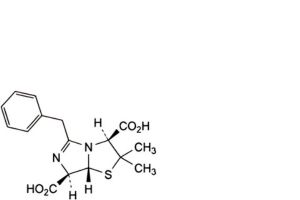
D. (3S,7R,7aR)-5-benzyl-2,2-dimethyl-2,3,7,7a-tetrahydroimidazo[5,1-b]thiazole-3,7-dicarboxylic acid (penillic acid of benzylpenicillin),
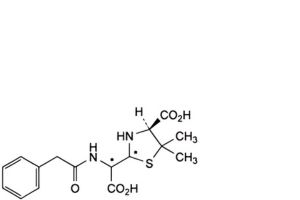
E. (4S)-2-[carboxy[(phenylacetyl)amino]methyl]-5,5-dimethylthiazolidine-4-carboxylic acid (penicilloic acids of benzylpenicillin),
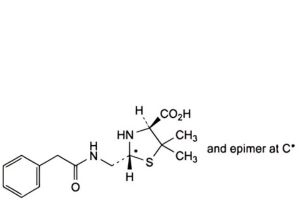
F. (2RS,4S)-5,5-dimethyl-2-[[(phenylacetyl)amino]methyl]thiazolidine-4-carboxylic acid (penilloic acids of benzylpenicillin),
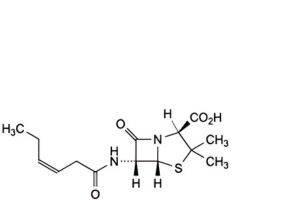
G. (2S,5R,6R)-6-[(3Z)-hex-3-enoylamino]-3,3-dimethyl-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylic acid,
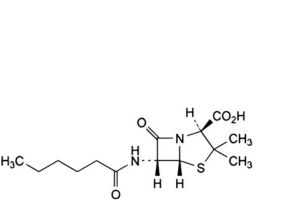
H. (2S,5R,6R)-6-(hexanoylamino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (dihydropenicillin F).