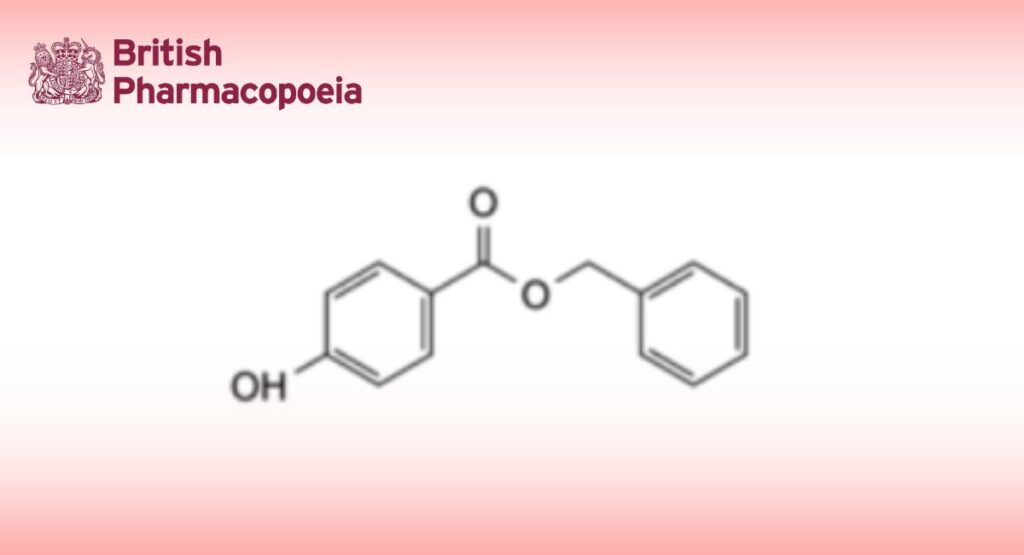
Benzylparaben
C14H12O3 228.3 94-18-8
Action and use
Antimicrobial preservative.
DEFINITION
Benzyl Hydroxybenzoate is benzyl 4-hydroxybenzoate. It contains not less than 99.0% and not more than 101.0% of C14H12O3.
CHARACTERISTICS
A white to creamy white, crystalline powder.
Practically insoluble in water; freely soluble in ethanol (96%) and in ether. It dissolves in solutions of alkali hydroxides.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of benzyl
hydroxybenzoate (RS 028).
B. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.001% w/v solution in ethanol (96%) exhibits a maximum only at 260 nm. The absorbance at the maximum at 260 nm is about 0.76.
C. Dissolve 0.1 g in 2 mL of ethanol (96%), boil and add 0.5 mL of nitric acid solution of mercury. A precipitate is produced slowly and the supernatant liquid becomes red.
D. Melting point, about 112°, Appendix V A.
TESTS
Acidity
Dissolve 0.2 g in 10 mL of ethanol (50%) previously neutralised to methyl red solution and titrate with 0.1M sodium hydroxide VS using methyl red solution as indicator. Not more than 0.1 mL of 0.1M sodium hydroxide VS is required to Benzyl Hydroxybenzoate change the colour of the solution.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using a plate precoated with silica gel F254, the surface of which has been modified with chemically-bonded octadecylsilyl groups (Whatman KC18F plates are suitable) and a mixture of 70 volumes of methanol, 30 volumes of water and 1 volume of glacial acetic acid as the mobile phase. Apply separately to the plate 2 μL of each of two solutions of the substance being examined in acetone containing (1) 1.0% w/v and (2) 0.010% w/v. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm). Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram
obtained with solution (2).
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Gently boil 0.12 g under a reflux condenser with 20 mL of 2M sodium hydroxide for 30 minutes. Cool and extract with three 20-mL quantities of 1,2-dichloroethane. Wash the combined extracts with 20 mL of 0.1M sodium hydroxide and add the washings to the main aqueous phase, discarding the organic layer. To the aqueous solution add 25 mL of 0.0333M potassium bromate VS, 5 mL of a 12.5% w/v solution of potassium bromide and 10 mL of hydrochloric acid and immediately stopper the flask. Shake for 15 minutes and allow to stand for 15 minutes. Add 25 mL of dilute potassium iodide solution and shake vigorously. Titrate the liberated iodine with 0.1M sodium thiosulfate VS using starch mucilage,
added towards the end of the titration, as indicator. Repeat the operation without the substance being examined. The difference between the titrations represents the amount of potassium bromate required. The volume of 0.0333M potassium bromate VS used is equivalent to half of the volume of 0.1M sodium thiosulfate VS required for the titration. Each mL of 0.0333M potassium bromate VS is equivalent to 7.608 mg of C14H12O3