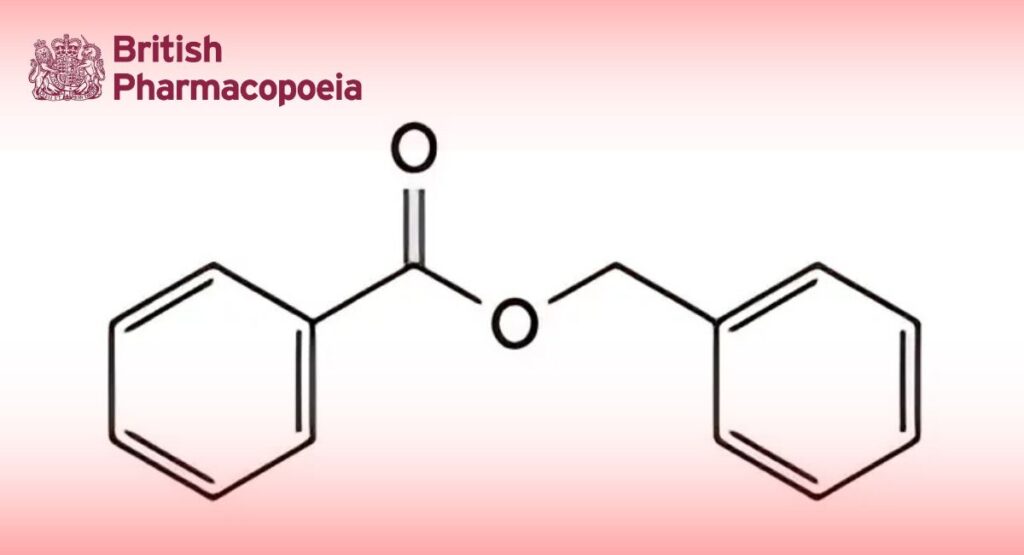
(Ph. Eur. monograph 0705)
C14H12O2 212.2 120-51-4
Action and use
Used topically in the treatment of scabies.
Preparation
Benzyl Benzoate Application
DEFINITION
Phenylmethyl benzoate.
Content
99.0 per cent to 100.5 per cent.
CHARACTERS
Appearance
Colourless or almost colourless crystals or colourless or almost colourless, oily liquid.
Solubility
Practically insoluble in water, miscible with ethanol (96 per cent), with methylene chloride and with fatty and essential oils.
bp
About 320 °C.
IDENTIFICATION
First identification: A.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of benzyl benzoate.
B. To 2 g add 25 mL of alcoholic potassium hydroxide solution R and boil under a reflux condenser for 2 h. Remove the ethanol on a water-bath, add 50 mL of water R and distill. Collect about 25 mL of distillate and use it for identification test C. Acidify the liquid remaining in the distillation flask with dilute hydrochloric acid R. A white precipitate is formed that, when washed with water R and dried in vacuo melts (2.2.14) at 121 °C to 124 °C.
C. To the distillate obtained in identification test B add 2.5 g of potassium permanganate R and 5 mL of dilute sodium hydroxide solution R. Boil under a reflux condenser for 15 min, cool and filter. Acidify the filtrate with dilute hydrochloric acid R. A white precipitate is formed that, when washed with water R and dried in vacuo, melts (2.2.14) at 121 °C to
124 °C.
TESTS
Acidity
Dissolve 2.0 g in ethanol (96 per cent) R and dilute to 10 mL with the same solvent. Titrate with 0.1 M sodium hydroxide using phenolphthalein solution R as indicator. Not more than 0.2 mL is required to change the colour of the indicator to pink.
Relative density (2.2.5)
1.118 to 1.122.
Refractive index (2.2.6)
1.568 to 1.570.
Freezing point (2.2.18)
Minimum 17.0 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
To 2.000 g add 50.0 mL of 0.5 M alcoholic potassium hydroxide and boil gently under a reflux condenser for 1 h. Titrate the hot solution with 0.5 M hydrochloric acid using 1 mL of phenolphthalein solution R as indicator. Carry out a blank determination.
1 mL of 0.5 M alcoholic potassium hydroxide is equivalent to 106.1 mg of C14H12O2.
STORAGE
In an airtight, well-filled container, protected from light.