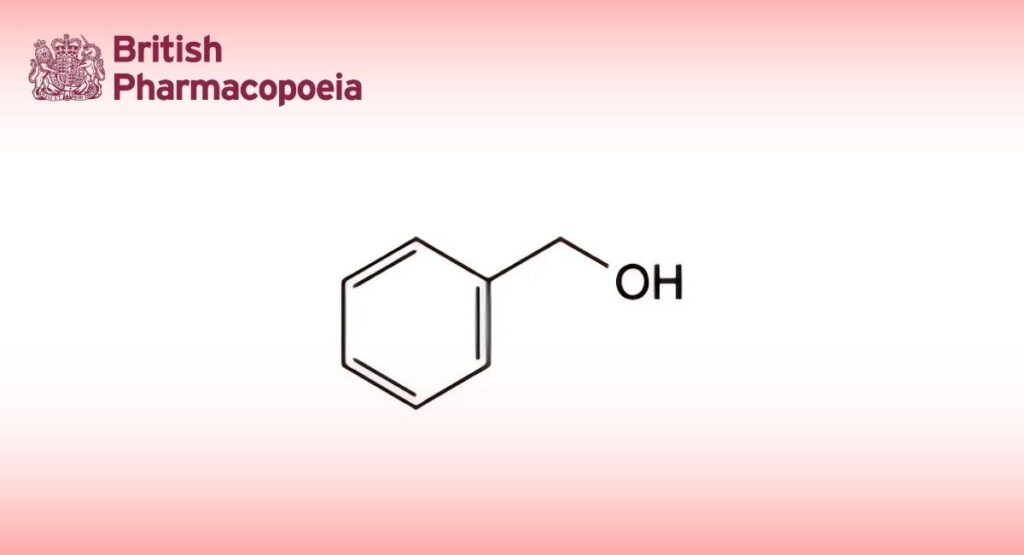
(Ph. Eur. monograph 0256)
C7H8O 108.1 100-51-6
Action and use
Local anaesthetic; disinfectant.
DEFINITION
Phenylmethanol.
Content
98.0 per cent to 100.5 per cent.
CHARACTERS
Appearance
Clear, colourless, oily liquid.
Solubility
Soluble in water, miscible with ethanol (96 per cent) and with fatty and essential oils.
Relative density
1.043 to 1.049.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison benzyl alcohol CRS.
TESTS
Appearance of solution
Shake 2.0 mL with 60 mL of water R. It dissolves completely. The solution is clear (2.2.1) and colourless (2.2.2, Method II).♦
Acidity
To 10 mL add 10 mL of ethanol (96 per cent) R and 1 mL of phenolphthalein solution R. Not more than 1 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator to pink.
Refractive index (2.2.6)
1.538 to 1.541.
Peroxide value (2.5.5, Method A)
Maximum 5.
Related substances
Gas chromatography (2.2.28).
Test solution: The substance to be examined.
Standard solution (a): Dissolve 0.100 g of ethylbenzene R in the test solution and dilute to 10.0 mL with the same solution. Dilute 2.0 mL of this solution to 20.0 mL with the test solution.
Standard solution (b): Dissolve 2.000 g of dicyclohexyl R in the test solution and dilute to 10.0 mL with the same solution. Dilute 2.0 mL of this solution to 20.0 mL with the test solution.
Reference solution (a): Dissolve 0.750 g of benzaldehyde R and 0.500 g of cyclohexylmethanol R in the test solution and dilute to 25.0 mL with the test solution. Add 1.0 mL of this solution to a mixture of 2.0 mL of standard solution (a) and 3.0 mL of standard solution (b) and dilute to 20.0 mL with the test solution.
Reference solution (b): Dissolve 0.250 g of benzaldehyde R and 0.500 g of cyclohexylmethanol R in the test solution and dilute to 25.0 mL with the test solution. Add 1.0 mL of this solution to a mixture of 2.0 mL of standard solution (a) and 2.0 mL of standard solution (b) and dilute to 20.0 mL with the test solution.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.32 mm;
— stationary phase: macrogol 20 000 R (film thickness 0.5 μm).
Carrier gas helium for chromatography R.
Linear velocity 25 cm/s at 50 °C.
Temperature:
| Time
(min) |
Temperature
(°C) |
|
| Column | 0 – 34 | 50 → 220 |
| 34 – 69 | 220 | |
| Injection port | 200 | |
| Detector | 310 |
Detection: Flame ionisation.
Benzyl alcohol not intended for parenteral administration
Injection: Without air-plug, 0.1 μL of the test solution and reference solution (a).
Relative retention: With reference to benzyl alcohol (retention time = about 26 min): ethylbenzene = about 0.28; dicyclohexyl = about 0.59; impurity A = about 0.68; impurity B = about 0.71.
System suitability Reference solution (a):
— resolution: minimum 3.0 between the peaks due to impurities A and B.
If any peaks in the chromatogram obtained with the test solution have the same retention time as the peaks due to ethyl benzene or dicyclohexyl, subtract the areas of any such peaks from the peak areas at these retention times in the chromatograms obtained with reference solutions (a) or (b) (corrected peak areas of ethyl benzene and dicyclohexyl). Any such peaks in the chromatogram obtained with the test solution are to be included in the assessments for the sum of other peaks.
Limits:
— impurity A: not more than the difference between the area of the peak due to impurity A in the chromatogram obtained with reference solution (a) and the area of the peak due to impurity A in the chromatogram obtained with the test solution (0.15 per cent);
— impurity B: not more than the difference between the area of the peak due to impurity B in the chromatogram obtained with reference solution (a) and the area of the peak due to impurity B in the chromatogram obtained with the test solution (0.10 per cent);
— sum of other peaks with a relative retention less than that of benzyl alcohol: not more than 4 times the area of the peak due to ethylbenzene in the chromatogram obtained with reference solution (a) corrected if necessary as described above (0.04 per cent);
— sum of peaks with a relative retention greater than that of benzyl alcohol: not more than the area of the peak due to dicyclohexyl in the chromatogram obtained with reference solution (a) corrected if necessary as described above (0.3 per cent);
— disregard limit: 0.01 times the area of the peak due to ethylbenzene in the chromatogram obtained with reference solution (a) corrected if necessary as described above (0.0001 per cent).
Benzyl alcohol intended for parenteral administration
Injection Without air-plug, 0.1 μL of the test solution and reference solution (b).
Relative retention With reference to benzyl alcohol (retention time = about 26 min): ethylbenzene = about 0.28; dicyclohexyl = about 0.59; impurity A = about 0.68; impurity B = about 0.71.
System suitability Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurities A and B.
If any peaks in the chromatogram obtained with the test solution have the same retention times as the peaks due to ethyl benzene or dicyclohexyl, subtract the areas of any such peaks from the peak areas at these retention times in the chromatograms obtained with reference solutions (a) or (b) (corrected peak areas of ethyl benzene and dicyclohexyl). Any such peaks in the chromatogram obtained with the test solution are to be included in the assessments for the sum of other peaks.
Limits:
— impurity A: not more than the difference between the area of the peak due to impurity A in the chromatogram obtained with reference solution (b) and the area of the peak due to impurity A in the chromatogram obtained with the test solution (0.05 per cent);
— impurity B: not more than the difference between the area of the peak due to impurity B in the chromatogram obtained with reference solution (b) and the area of the peak due to impurity B in the chromatogram obtained with the test solution (0.10 per cent);
— sum of other peaks with a relative retention less than that of benzyl alcohol: not more than twice the area of the peak due to ethylbenzene in the chromatogram obtained with reference solution (b) corrected if necessary as described above (0.02 per cent);
— sum of peaks with a relative retention greater than that of benzyl alcohol: not more than the area of the peak due to dicyclohexyl in the chromatogram obtained with reference solution (b) corrected if necessary as described above (0.2 per cent);
— disregard limit: 0.01 times the area of the peak due to ethylbenzene in the chromatogram obtained with reference solution (b) corrected if necessary as described above (0.0001 per cent).
Residue on evaporation
Maximum 0.05 per cent.
After ensuring that the substance to be examined complies with the test for peroxide value, evaporate 10.0 g to dryness in a tared quartz or porcelain crucible or platinum dish on a hot plate at a temperature not exceeding 200 °C. Ensure that the substance to be examined does not boil during evaporation. Dry the residue on the hot plate for 1 h and allow to cool in a desiccator. The residue weighs a maximum of 5 mg.
ASSAY
To 0.900 g (m g) add 15.0 mL of a freshly prepared mixture of 1 volume of acetic anhydride R and 7 volumes of anhydrous pyridine R and heat under a reflux condenser on awater-bath for 30 min. Cool and add 25 mL of water R. Using 0.25 mL of phenolphthalein solution R as indicator, titrate with 1 M sodium hydroxide (n1 mL). Carry out a blank titration (n2 mL).
10.81(n1 – n2)/m
Calculate the percentage content of C7H8O using the following expression:
STORAGE
In an airtight container, under nitrogen, protected from light and at a temperature between 2 °C and 8 °C.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
Specified impurities A, B.
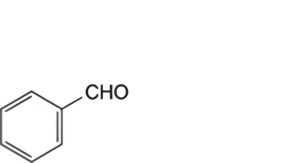
A. benzaldehyde,
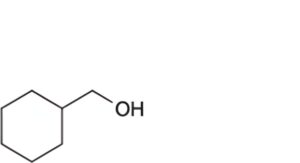
B. cyclohexylmethanol.
This monograph has undergone pharmacopoeial harmonisation. See chapter 5.8. Pharmacopoeial harmonisation.