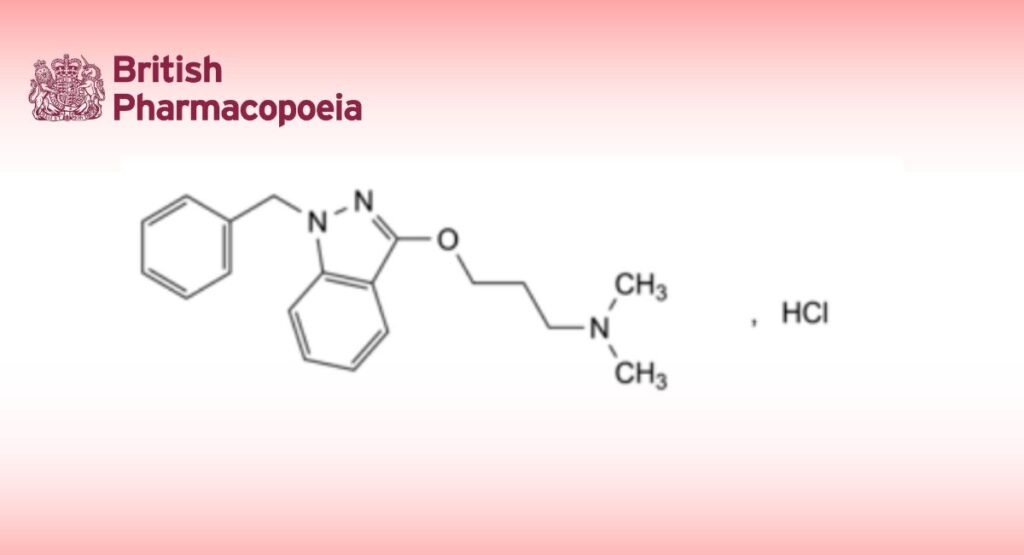
(Ph. Eur. monograph 2759)
C19H24ClN3O 345.9 132-69-4
Action and use
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
Preparations
Benzydamine Cream
Benzydamine Mouthwash
Benzydamine Oromucosal Spray
DEFINITION
3-[(1-Benzyl-1H-indazol-3-yl)oxy]-N,N-dimethylpropan-1-amine hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, hygroscopic, crystalline powder.
Solubility
Very soluble in water, freely soluble in ethanol (96 per cent) and in methylene chloride, slightly soluble in acetone, practically insoluble in heptane.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison benzydamine hydrochloride CRS.
B. It gives reaction (a) of chlorides (2.3.1).
TESTS
Impurity G
Liquid chromatography (2.2.29) coupled with mass spectrometry (2.2.43).
Solvent mixture: Mobile phase A, mobile phase B (8:92 V/V).
Test solution: Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution: Dissolve 5.0 mg of benzydamine impurity G CRS in methanol R and dilute to 1000.0 mL with methanol R. Dilute 1.0 mL of this solution to 1000.0 mL with the solvent mixture.
Column:
— size: l = 0.10 m, Ø = 3.0 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography compatible with 100 per cent aqueous mobile phases R (2.5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: in 1000 mL of methanol R3 mix 500 μL of formic acid R and 130 μL of heptafluorobutyric acid R;
— mobile phase B: in 1000 mL of water for chromatography R mix 500 μL of formic acid R and 130 μL of heptafluorobutyric acid R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 9 | 8 | 92 |
| 9 – 15 | 8 → 26 | 92 → 74 |
| 15 – 15.1 | 26 → 80 | 74 → 20 |
| 15.1 – 20 | 80 | 20 |
Flow rate 0.3 mL/min.
Detection Mass detector: the following settings have been found to be suitable for triple-quadrupole mass spectrometer and are given as examples; if the detector has different setting parameters, adjust the detector settings so as to comply with the system suitability criteria:
— ionisation: ESI-positive;
— ion spray voltage: 5.5 kV;
— ion source temperature: 350 °C;
— declustering potential: 67 V;
— Q1/Q3 resolution: unit resolution;
— dwell time: 500 ms;
— acquisition starting time: 4 min;
— acquisition ending time: 16 min;
— ion transition: 122.1 m/z to 46.0 m/z;
— collision energy: 30 eV.
Injection 6 μL.
Retention time: Impurity G = about 7 min.
System suitability: Reference solution:
— repeatability: maximum relative standard deviation of 5.0 per cent for the area of the peak due to impurity G determined on 6 injections using the extracted ion chromatogram showing the total ion current of the ion transition used for quantification.
Calculation of content:
— use the concentration of impurity G in the reference solution.
Limit:
— impurity G: maximum 5 ppm.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture methanol R, water R (50:50 V/V).
Test solution: Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b) Dissolve 5 mg of benzydamine for system suitability CRS (containing impurities A, B and D) in 5 mL of the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: amidoalkylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: dissolve 1.36 g of potassium dihydrogen phosphate R in 900 mL of water for chromatography R, adjust to pH 3.0 with phosphoric acid R, add 1.16 g of sodium octanesulfonate R and dilute to 1000 mL with water for chromatography R;
— mobile phase B: methanol R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 50 | 50 |
| 2 – 22 | 50 → 35 | 50 → 65 |
| 22 – 29 | 35 | 65 |
Flow rate 1.5 mL/min.
Detection: Spectrophotometer at 320 nm.
Equilibration: At least 10 min with the mobile phase at the initial composition.
Injection 20 μL.
Identification of impurities: Use the chromatogram supplied with benzydamine for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B and D.
Relative retention: With reference to benzydamine (retention time = about 12 min): impurity A = about 1.36; impurity D = about 1.43; impurity B = about 2.0.
System suitability:
— resolution: minimum 1.5 between the peaks due to impurities A and D in the chromatogram obtained with reference solution (b);
— signal-to-noise ratio: minimum 38 for the principal peak in the chromatogram obtained with reference solution (a). Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 1.9; impurity D = 1.4;
— for each impurity, use the concentration of benzydamine hydrochloride in reference solution (a).
Limits:
— impurity B: maximum 0.5 per cent;
— impurity A: maximum 0.2 per cent;
— impurity D: maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 1.0 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in 50 mL of ethanol (96 per cent) R. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M sodium hydroxide is equivalent to 34.59 mg of C19H24ClN3O.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B, D, G.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) C, E, F.
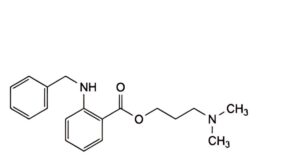
A. 3-(dimethylamino)propyl 2-(benzylamino)benzoate,
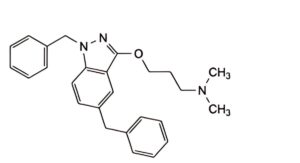
B. 3-[(1,5-dibenzyl-1H-indazol-3-yl)oxy]-N,N-dimethylpropan-1-amine,
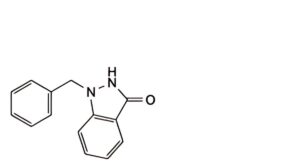
C. 1-benzyl-1,2-dihydro-3H-indazol-3-one,
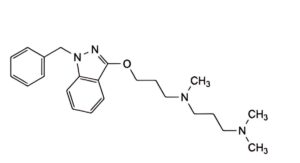
D. N -[3-[(1-benzyl-1H-indazol-3-yl)oxy]propyl]-N ,N ,N -trimethylpropane-1,3-diamine,
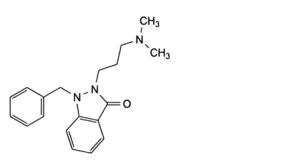
E. 1-benzyl-2-[3-(dimethylamino)propyl]-1,2-dihydro-3H-indazol-3-one,
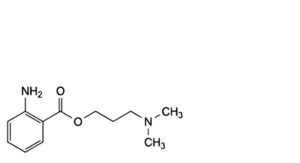
F. 3-(dimethylamino)propyl 2-aminobenzoate,
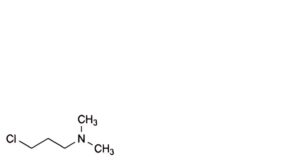
G. 3-chloro-N,N-dimethylpropan-1-amine.