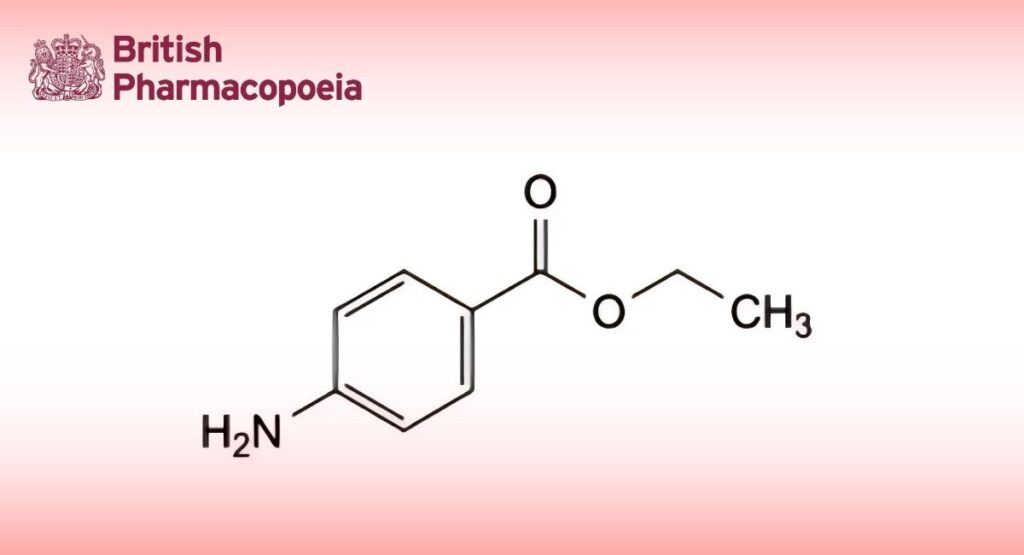
(Ph. Eur. monograph 0011)
C9H11NO2 165.2 94-09-7
Action and use
Local anaesthetic.
DEFINITION
Ethyl 4-aminobenzoate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals.
Solubility
Very slightly soluble in water, freely soluble in ethanol (96 per cent).
It shows polymorphism (5.9).
IDENTIFICATION
First identification: A.
Second identification: B.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: benzocaine CRS.
If the spectra obtained show differences, dissolve the substance to be examined and the reference substance separately in anhydrous ethanol R, evaporate to dryness and record new spectra using the residues.
B. Melting point (2.2.14).
Determination A Determine the melting point of the substance to be examined.
Result A 89 °C to 92 °C.
Determination B: Mix equal parts of the substance to be examined and benzocaine CRS and determine the melting point of the mixture.
Result B: The absolute difference between the melting point of the mixture and the value obtained in determination A is not greater than 2 °C.
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture acetonitrile R1, water for chromatography R (50:50 V/V).
Test solution: Dissolve 25.0 mg of the substance to be examined in 5 mL of acetonitrile R and dilute to 50.0 mL with the solvent mixture.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b): Dissolve 5 mg of the substance to be examined and 5 mg of 4-nitrobenzoic acid R (impurity E) in 10 mL of the solvent mixture. Dilute 1 mL of the solution to 50 mL with the solvent mixture.
Column:
— size: l = 0.10 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography compatible with 100 per cent aqueous mobile phases R (3 μm);
— temperature: 35 °C.
Mobile phase:
— mobile phase A: dilute 1 mL of perchloric acid R to 100 mL with water for chromatography R; dilute 1 mL of this solution to 100 mL with water for chromatography R; mix 9 volumes of this solution and 1 volume of acetonitrile R1;
— mobile phase B: acetonitrile R1;
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 2 | 100 | 0 |
| 2 – 15 | 100 → 38.5 | 0 → 61.5 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 10 μL.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity E.
Relative retention: With reference to benzocaine (retention time = about 10 min): impurity E = about 0.9.
System suitability: Reference solution (b):
— resolution: minimum 5.0 between the peaks due to impurity E and benzocaine.
Calculation of percentage contents:
— for each impurity, use the concentration of benzocaine in reference solution (a).
Limits:
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.2 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Carry out the determination of primary aromatic amino-nitrogen (2.5.8), using 0.400 g dissolved in a mixture of 25 mL of hydrochloric acid R and 50 mL of water R.
1 mL of 0.1 M sodium nitrite is equivalent to 16.52 mg of C9H11NO2.
STORAGE
Protected from light.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, G, H.
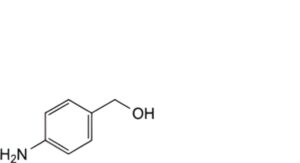
A. (4-aminophenyl)methanol,
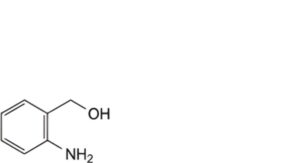
B. (2-aminophenyl)methanol,
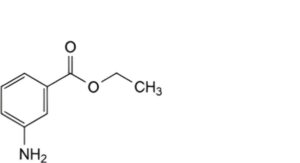
C. ethyl 3-aminobenzoate,
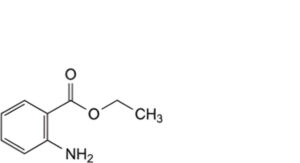
D. ethyl 2-aminobenzoate,
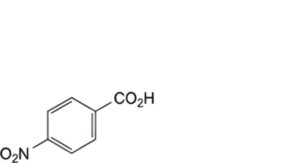
E. 4-nitrobenzoic acid,
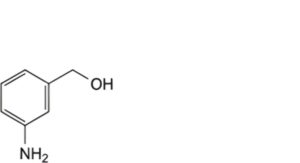
F. (3-aminophenyl)methanol,
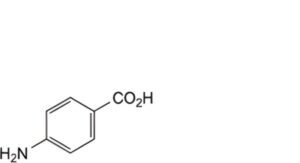
G. 4-aminobenzoic acid,
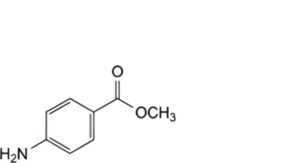
H. methyl 4-aminobenzoate.