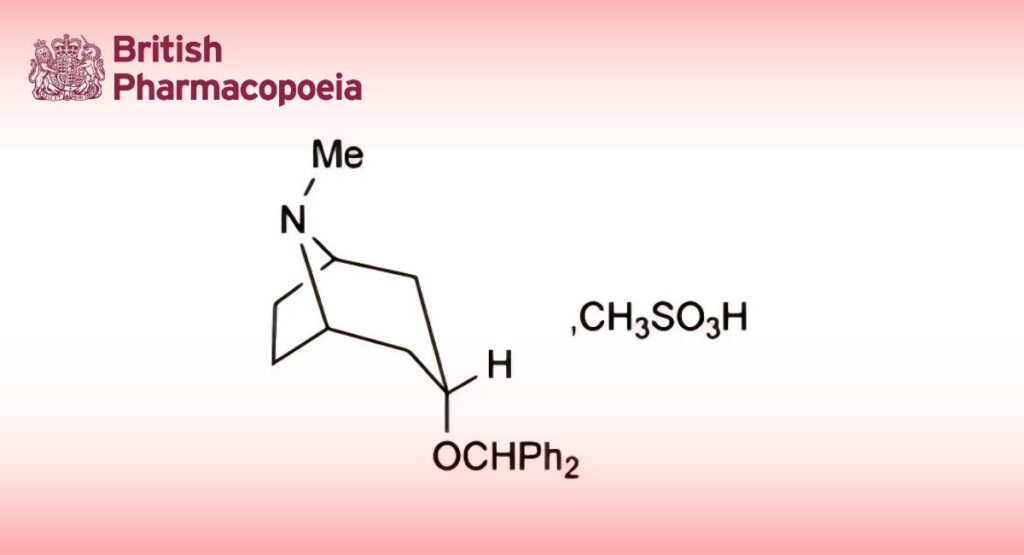
C21H25NO,CH4O3S 403.5 132-17-2
Action and use
Anticholinergic.
Preparations
Benzatropine Injection
Benzatropine Tablets
DEFINITION
Benzatropine Mesilate is (1R,3R,5S)-3-benzhydryloxytropane methanesulfonate. It contains not less than 98.0% and not more than 100.5% of C21H25NO,CH4O3S, calculated with reference to the dried substance.
PRODUCTION
Risk assessment should be used to evaluate the potential for genotoxic methanesulfonate esters to be formed in the presence of low molecular weight alcohols. If a risk of methanesulfonate ester formation is identified through risk assessment, these impurities should not exceed the threshold of toxicological concern.
CHARACTERISTICS
A white, crystalline powder. It melts at about 144°.
Very soluble in water; freely soluble in ethanol (96%); practically insoluble in ether.
IDENTIFICATION
A. Dry the substance at 105° for 3 hours. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of benzatropine mesilate (RS 026).
B. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.1% w/v solution in 2M hydrochloric acid exhibits two maxima, at 253 and 258 nm. The absorbance at 253 nm is about 0.96 and at 258 nm is about 1.1.
C. Dissolve 10 mg in 2 mL of water, pour into 5 mL of hot picric acid solution R1 and allow to cool. The melting point of the precipitate, after drying at 105°, is about 185°, Appendix V A.
TESTS
Tropine
Carry out the method for thin-layer chromatography, Appendix III A, using silica gel G as the coating substance and a mixture of 75 volumes of ethanol (96%) and 15 volumes of 13.5M ammonia as the mobile phase. Apply separately to the plate 10 μL of each of two solutions in acetone containing (1) 4.0% w/v of the substance being examined and (2) 0.020% w/v of tropine. After removal of the plate, allow it to dry in air and spray with sodium iodobismuthate solution and then with a 0.4% w/v solution of sulfuric acid. Any spot corresponding to tropine in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2).
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. For solution (1) mix with the aid of ultrasound 50 mg of the substance being examined with 15 mL of mobile phase A, dilute to 50 mL with the same solvent and filter. For solution (2) dilute 1 volume of solution (1) to 100 volumes with mobile phase A and further dilute 1 volume of the resulting solution to 5 volumes with the same solvent. For solution (3) mix with the aid of ultrasound 50 mg of desmethyl benzatropine hydrochloride BPCRS with 15 mL of mobile phase A, dilute to 100 mL and dilute 1 volume of the resulting solution to 100 volumes with the same solvent. Solution (4) contains 0.01% w/v each of benzatropine mesilate BPCRS and desmethyl benzatropine hydrochloride BPCRS in mobile phase A.
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 4.6 mm) packed with phenylsilyl silica gel for chromatography (5 μm) (Zorbax SB-Phenyl 5μ is suitable). Carry out a linear gradient elution with a flow rate of 1 mL per minute using the following conditions. Use a detection wavelength of 220 nm.
Mobile phase A: A mixture of 5 volumes of a 1M potassium phosphate buffer prepared as described for mobile phase B, 20 volumes of acetonitrile and 75 volumes of water.
Mobile phase B: A mixture of 35 volumes of water, 60 volumes of acetonitrile and 5 volumes of a 1M potassium phosphate buffer prepared in the following manner: dissolve 136.1 g of potassium dihydrogen orthophosphate in 900 mL of water, add 5 mL of orthophosphoric acid (85%) and dilute to 1000 mL.
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
Comment
|
| 0 – 20 | 70 → 30 | 30 → 70 | linear gradient |
| 20 – 30 | 30 → 0 | 70 → 100 | linear gradient |
| 30 – 55 | 0 | 100 | isocratic |
| 55 – 65 | 70 | 30 | isocratic |
Inject: 20 μL of solution (4). The test is not valid unless the resolution factor between the two principal peaks is at least 1. If necessary adjust the concentration of acetonitrile or adjust the time program of the linear gradient elution.
Inject separately: 20 μL of mobile phase A as a blank and 20 μL each of solutions (1), (2) and (3). In the chromatogram obtained with solution (1) the area of any peak corresponding to desmethyl benzatropine is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (0.5%), the area of any other secondary peak is not greater that the area of the principal peak in the chromatogram obtained with solution (2) (0.2%) and the sum of the areas of any such peaks is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%). In solution (1) disregard any peaks corresponding to the peaks in the chromatogram obtained with the blank solution.
Loss on drying
When dried to constant weight at 105°, loses not more than 5.0% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.6 g in 25 mL of water, add 5 mL of dilute sodium carbonate solution and extract with four 10 mL quantities of chloroform. Wash the combined extracts with 10 mL of water, extract the washings with 5 mL of chloroform and add the chloroform to the combined extracts. Filter and wash the filter with 5 mL of chloroform. To the combined filtrate and washings add 25 mL of 1,4-dioxan and titrate with 0.1M perchloric acid VS using 0.15 mL of a 0.1% w/v solution of methyl red in methanol as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 40.35 mg of C21H25NO,CH4O3S.