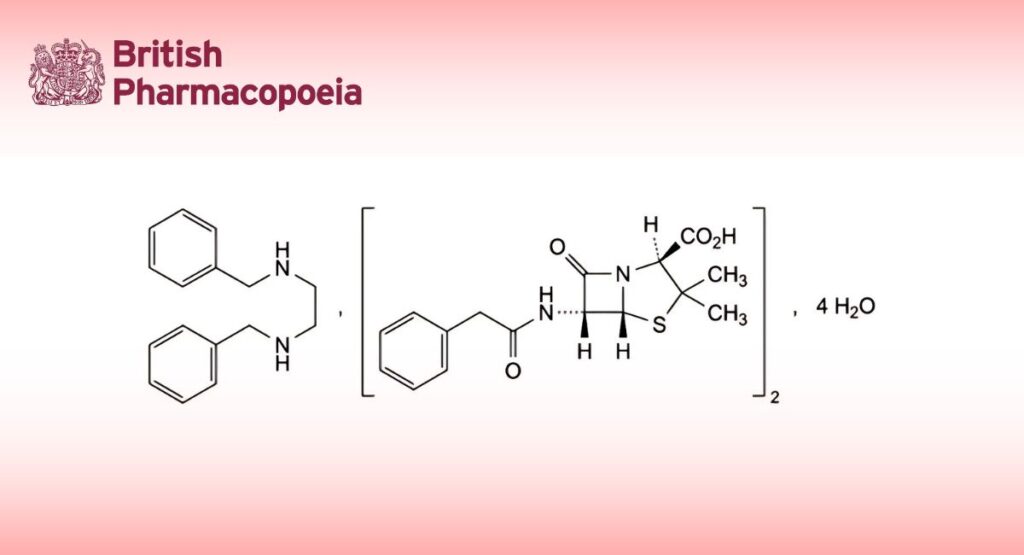
Benzathine Benzylpenicillin
(Benzylpenicillin (Benzathine) Tetrahydrate, Ph. Eur. monograph 0373)
C48H56N6O8S2,4H2O 981 41372-02-5
Action and use
Penicillin antibacterial.
DEFINITION
N ,N -Dibenzylethane-1,2-diamine bis[(2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-
azabicyclo[3.2.0]heptane-2-carboxylate] tetrahydrate.
Salt obtained from Benzylpenicillin sodium (0114) or Benzylpenicillin potassium (0113) produced by the growth of certain strains of Penicillium notatum or related micro-organisms.
Content
— benzathine benzylpenicillin: 94.5 per cent to 102.0 per cent (anhydrous substance) without correction for dispersing or suspending agents;
— benzathine: 24.0 per cent to 27.0 per cent (anhydrous substance).
Dispersing or suspending agents (e.g. lecithin and polysorbate 80) may be added.
CHARACTERS
Appearance
White or almost white, slightly hygroscopic powder.
Solubility
Very slightly soluble in water, freely soluble in dimethylformamide and in formamide, slightly soluble in ethanol (96 percent).
IDENTIFICATION
First identification: A.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison benzathine benzylpenicillin CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 25 mg of the substance to be examined in 5 mL of methanol R.
Reference solution: Dissolve 25 mg of benzathine benzylpenicillin CRS in 5 mL of methanol R.
Plate: TLC silanised silica gel plate R.
Mobile phase: Mix 30 volumes of acetone R and 70 volumes of a 154 g/L solution of ammonium acetate R previously adjusted to pH 7.0 with ammonia R.
Application: 1 μL.
Development: Over 2/3 of the plate.
Drying: In air.
Detection: Expose to iodine vapour until the spots appear and examine in daylight.
System suitability: Reference solution:
— the chromatogram shows 2 clearly separated spots.
Results: The 2 principal spots in the chromatogram obtained with the test solution are similar in position, colour and size to the 2 principal spots in the chromatogram obtained with the reference solution.
C. Place about 2 mg in a test-tube about 150 mm long and 15 mm in diameter. Moisten with 0.05 mL of water R and add 2 mL of sulfuric acid-formaldehyde reagent R. Mix the contents of the tube by swirling; the solution is practically colourless.Place the test-tube on a water-bath for 1 min; a reddish-brown colour develops.
D. To 0.1 g add 2 mL of 1 M sodium hydroxide and shake for 2 min. Shake the mixture with 2 quantities, each of 3 mL, of ether R. Evaporate the combined ether layers to dryness and dissolve the residue in 1 mL of ethanol (50 per cent V/V) R.
Add 5 mL of picric acid solution R, heat at 90 °C for 5 min and allow to cool slowly. Separate the crystals and recrystallise from ethanol (25 per cent V/V) R containing 10 g/L of picric acid R. The crystals melt (2.2.14) at about 214 °C.
TESTS
Acidity or alkalinity
To 0.50 g add 100 mL of carbon dioxide-free water R and shake for 5 min. Filter through a sintered-glass filter (2.1.2). To 20 mL of the filtrate add 0.1 mL of bromothymol blue solution R1. The solution is green or yellow. Not more than 0.2 mL of 0.02 M sodium hydroxide is required to change the colour of the indicator to blue.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use and by diluting to volume immediately after dissolution.
Solution A: Prepare a solution containing 1.3 g/L of disodium hydrogen phosphate dodecahydrate R and 6.8 g/L of potassium dihydrogen phosphate R.
Test solution (a): Dissolve 40.0 mg of the substance to be examined in 50 mL of methanol R and dilute to 100.0 mL with solution A.
Test solution (b): Dissolve 70.0 mg of the substance to be examined in 25 mL of methanol R and dilute to 50.0 mL with solution A.
Reference solution (a): Dissolve 40.0 mg of benzathine benzylpenicillin CRS in 50 mL of methanol R and dilute to 100.0 mL with solution A.
Reference solution (b): Dissolve 3 mg of benzathine benzylpenicillin for peak identification CRS (containing impurities A,B, C, D, E, F, G, H, I, J and K) in 1 mL of methanol R and dilute to 2 mL with solution A.
Reference solution (c): Dilute 1.0 mL of reference solution (a) to 20.0 mL with a mixture of equal volumes of methanol R and solution A.
Reference solution (d) Dilute 3.0 mL of reference solution (c) to 100.0 mL with a mixture of equal volumes of methanol R and solution A.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 50 °C.
Mobile phase:
— mobile phase A: mix 10 volumes of a 34 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 3.3 with phosphoric acid R, 30 volumes of methanol R1 and 60 volumes of water for chromatography R;
— mobile phase B: mix 5 volumes of a 34 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 3.3 with phosphoric acid R, 25 volumes of water for chromatography R and 70 volumes of methanol R1;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 85 | 15 |
| 2 – 16 | 85 → 0 | 15 → 100 |
| 16 – 26 | 0 | 100 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 220 nm.
Injection: 20 μL of test solution (b) and reference solutions (b), (c) and (d).
Identification of impurities: Use the chromatogram supplied with benzathine benzylpenicillin for peak identification CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B, C, D, E, F, G, H, I, J and K.
Relative retention: With reference to benzylpenicillin (retention time = about 7 min): impurity A = about 0.18; benzathine = about 0.30; impurity D = about 0.36; impurity G = about 0.38; impurity J = about 0.44; impurity E = about 0.51 and 0.60; impurity B = about 0.69; impurity F = about 0.84 and 0.88; impurity H = about 1.22; impurity I = about 1.42; impurity C = about 1.75; impurity K = about 2.90.
System suitability:
— resolution: minimum 1.0 between the peaks due to the epimers of impurity F and minimum 1.5 between the peaks due to impurities D and G in the chromatogram obtained with reference solution (b);
— signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (d).
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity E = 1.9; impurity F = 1.5;
— for each impurity, use the concentration of benzylpenicillin in reference solution (c).
Limits:
— impurity C: maximum 2.0 per cent;
— impurity K: maximum 1.0 per cent;
— impurity J: maximum 0.5 per cent;
— impurities E (sum of isomers), F (sum of epimers): for each impurity, maximum 0.3 per cent;
— impurities A, B, D, G, H, I: for each impurity, maximum 0.2 per cent;
— any other impurity: for each impurity, maximum 0.2 per cent;
— total: maximum 3.5 per cent;
— reporting threshold: 0.05 per cent; disregard the peak due to benzathine.
Water (2.5.12)
5.0 per cent to 8.0 per cent, determined on 0.200 g.
Bacterial endotoxins (2.6.14, Method E)
Less than 0.13 IU/mL, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Suspend 20 mg in 20 mL of a solution of 0.1 M sodium hydroxide diluted 1 to 100, shake thoroughly and centrifuge. Examine the supernatant.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase: Mobile phase B, mobile phase A (15:85 V/V).
Injection: Test solution (a) and reference solution (a).
Calculate the percentage contents of benzathine (C16H20N2) and benzathine benzylpenicillin (C48H56N6O8S2) taking into account the assigned content of benzathine benzylpenicillin CRS.
STORAGE
In an airtight container. If the substance is sterile, the container is also sterile and tamper-evident.
IMPURITIES
Specified impurities A, B, C, D, E, F, G, H, I, J, K.
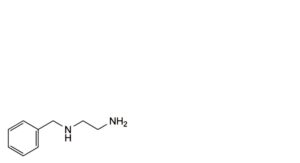
A. N -benzylethane-1,2-diamine,
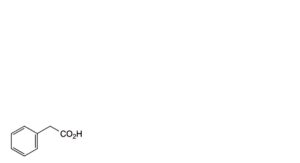
B. phenylacetic acid,
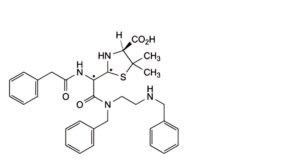
C. (2Ξ,4S)-2-[(1Ξ)-2-[benzyl[2-(benzylamino)ethyl]amino]-2-oxo-1-(2-phenylacetamido)ethyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid (benzylpenicilloic acids benzathide),
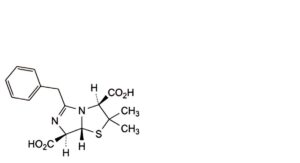
D. (3S,7R,7aR)-5-benzyl-2,2-dimethyl-2,3,7,7a-tetrahydroimidazo[5,1-b][1,3]thiazole-3,7-dicarboxylic acid (penillic acid of benzylpenicillin),
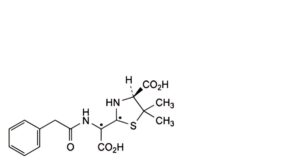
E. (2Ξ,4S)-2-[(Ξ)-carboxy(2-phenylacetamido)methyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid (penicilloic acids of benzylpenicillin),
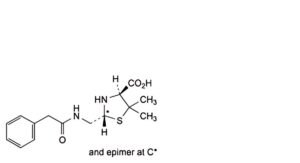
F. (2RS,4S)-5,5-dimethyl-2-[(2-phenylacetamido)methyl]-1,3-thiazolidine-4-carboxylic acid (penilloic acids of benzylpenicillin),
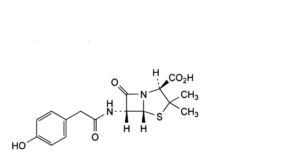
G. (2S,5R,6R)-6-[2-(4-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,
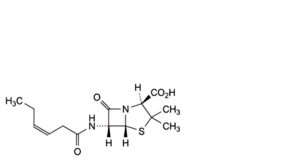
H. (2S,5R,6R)-6-[(3Z)-hex-3-enamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (iso-penicillin F),
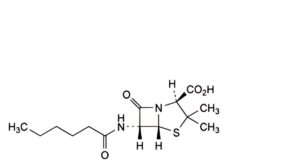
I. (2S,5R,6R)-6-hexanamido-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (dihydropenicillin F),
J. unknown structure,
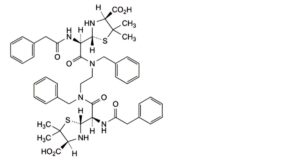
K. (2R,2′R,4S,4′S)-2,2′-[(4R,11R)-6,9-dibenzyl-2,5,10,13-tetraoxo-1,14-diphenyl-3,6,9,12-tetraazatetradecane-4,11- diyl]bis(5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid).