(Ph. Eur. monograph 0371)
Action and use
Antiseptic.
DEFINITION
Aqueous solution of a mixture of alkylbenzyldimethylammonium chlorides, the alkyl groups mainly having chain lengths of C12, C14 and C16.
Content
475 g/L to 525 g/L of alkylbenzyldimethylammonium chlorides, calculated using the average relative molecular mass (see Tests). The solution may contain ethanol (96 per cent).
CHARACTERS
Appearance
Clear, colourless or slightly yellowish liquid.
Solubility
Miscible with water and with ethanol (96 per cent).
It froths copiously when shaken.
IDENTIFICATION
First identification: B, E.
Second identification: A, C, D, E.
A. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution: Dilute 0.3 mL to 100.0 mL with water R.
Spectral range: 220-350 nm.
Absorption maxima: At 257 nm, 263 nm and 269 nm.
Shoulder: At about 250 nm.
B. Examine the chromatograms obtained in the test for average relative molecular mass and ratio of alkyl components.
Results: The principal peaks in the chromatogram obtained with the test solution are similar in retention time to the principal peaks in the chromatogram obtained with the reference solution.
C. To 0.05 mL add 2 mL of water R, 0.1 mL of glacial acetic acid R and, dropwise, 1 mL of sodium tetraphenylborate solution R. A white precipitate is formed. Filter. Dissolve the precipitate in a mixture of 1 mL of acetone R and 5 mL of ethanol (96 per cent) R, heating to not more than 70 °C. Add water R dropwise to the warm solution until a slight opalescence forms. Heat gently until the solution is clear and allow to cool. White crystals separate. Filter, wash with 3 quantities, each of 10 mL, of water R and dry in vacuo (2.2.32) at a temperature not exceeding 50 °C. The crystals melt (2.2.14) at 127 °C to 133 °C.
D. To 5 mL of dilute sodium hydroxide solution R add 0.1 mL of bromophenol blue solution R1 and 5 mL of methylene chloride R and shake. The methylene chloride layer is colourless. Add 0.05 mL of the solution to be examined and shake. The methylene chloride layer becomes blue.
E. To 0.05 mL add 1 mL of dilute nitric acid R. A white precipitate is formed which dissolves on the addition of 5 mL of ethanol (96 per cent) R. The solution gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dilute 2.0 g to 100 mL with carbon dioxide-free water R.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Acidity or alkalinity
To 50 mL of solution S add 0.1 mL of bromocresol purple solution R. Not more than 0.1 mL of 0.1 M hydrochloric acid or 0.1 M sodium hydroxide is required to change the colour of the indicator.
Average relative molecular mass and ratio of alkyl components
Liquid chromatography (2.2.29).
Test solution: Determine the density (2.2.5) of the solution to be examined. Dilute a quantity of the solution to be examined equivalent to about 0.400 g of benzalkonium chloride to 100.0 mL with water R.
Reference solution: Dissolve the contents of a vial of benzalkonium chloride for system suitability CRS in 5 mL of water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped cyanosilyl silica gel for chromatography R (5 μm).
Mobile phase: Mix 45 volumes of acetonitrile R and 55 volumes of a 13.6 g/L solution of sodium acetate R previously adjusted to pH 5.0 with glacial acetic acid R.
Flow rate: 2.0 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 10 μL.
Identification of homologues: Use the chromatogram supplied with benzalkonium chloride for system suitability CRS and the chromatogram obtained with the reference solution to identify the peaks due to homologues C12, C14 and C16.
Relative retention: With reference to C12 homologue (retention time = about 6 min): C14 homologue = about 1.3; C16 homologue = about 1.7.
System suitability: Reference solution:
— resolution: minimum 1.5 between the peaks due to the C12 and C14 homologues.
Calculate the average relative molecular mass of the sample by summing the products for each homologue, using the following expression:
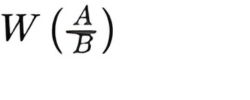
A = area of the peak due to the given homologue in the chromatogram obtained with the test solution;
B = sum of the areas of the peaks due to all homologues in the chromatogram obtained with the test solution;
W = relative molecular mass for the given homologue: 340, 368 and 396 for the C12, C14 and C16 homologues, respectively.
Calculate the percentage of each homologue, using the following expression:
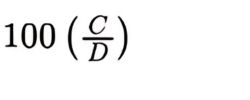
C = product of the relative molecular mass of the given homologue and the area of the corresponding peak in the chromatogram obtained with the test solution;
D = sum of the C values for all homologues quantified.
Limits:
— C12 homologue: minimum 40 per cent;
— C14 homologue: minimum 20 per cent;
— sum of C12 and C14 homologues: minimum 70 per cent.
Impurities A, B and C
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution Determine the density (2.2.5) of the solution to be examined. Dilute a quantity of the solution to be examined equivalent to 2.5 g of benzalkonium chloride to 50.0 mL with methanol R.
Reference solution (a): Dissolve 25.0 mg of benzyl alcohol CRS (impurity A) in methanol R and dilute to 100.0 mL with the same solvent.
Reference solution (b): Dissolve 75.0 mg of benzaldehyde CRS (impurity B) in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 10.0 mL with methanol R.
Reference solution (c:) Dilute 1 mL of reference solution (a) to 10 mL with methanol R.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: dissolve 1.09 g of sodium hexanesulfonate R and 6.9 g of sodium dihydrogen phosphate monohydrate R in water for chromatography R; adjust to pH 3.5 with phosphoric acid R and dilute to 1000 mL with the same solvent;
— mobile phase B: methanol R2;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 10 | 80 | 20 |
| 10 – 14 | 80 → 50 | 20 → 50 |
| 14 – 35 | 50 | 50 |
| 35 – 36 | 50 → 20 | 50 → 80 |
| 36 – 55 | 20 | 80 |
Flow rate:1.0 mL/min.
Detection: Spectrophotometer at 210 nm for impurities A and C, and at 257 nm for impurity B.
Injection: 20 μL.
Relative retention: With reference to impurity A (retention time = about 10 min): impurity B = about 1.3; impurity C = about 2.4.
System suitability: At 210 nm:
— signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (c);
— symmetry factor: minimum 0.6 for the peak due to impurity A in the chromatogram obtained with reference solution (a).
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity C by 1.3;
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— impurity C: not more than 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Amines and amine salts
Mix 10.0 g, while heating, with 20 mL of a mixture of 3 volumes of 1 M hydrochloric acid and 97 volumes of methanol R and add 100 mL of 2-propanol R. Pass a stream of nitrogen R slowly through the solution. Titrate with up to 12.0 mL of 0.1 M tetrabutylammonium hydroxide and record the potentiometric titration curve (2.2.20). If the curve shows 2 points of inflexion, the volume of titrant added between the 2 points is not greater than 5.0 mL. If the curve shows no point of inflexion, the solution to be examined does not comply with the test. If the curve shows 1 point of inflexion, repeat the test but add 3.0 mL of a 25.0 g/L solution of dimethyldecylamine R in 2-propanol R before the titration. If the titration curve after the addition of 12.0 mL of the titrant shows only 1 point of inflexion, the solution to be examined does not comply with the test.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Determine the density (2.2.5) of the solution to be examined. Dilute 4.00 g to 100.0 mL with water R. Transfer 25.0 mL of the solution to a separating funnel, add 25 mL of methylene chloride R, 10 mL of 0.1 M sodium hydroxide and 10.0 mL of a freshly prepared 50 g/L solution of potassium iodide R. Shake well, allow to separate and discard the methylene chloride layer. Shake the aqueous layer with 3 quantities, each of 10 mL, of methylene chloride R and discard the methylene chloride layers. To the aqueous layer add 40 mL of hydrochloric acid R, allow to cool and titrate with 0.05 M potassium iodate until the deep-brown colour is almost discharged. Add 5 mL of methylene chloride R and continue the titration, shaking vigorously, until the methylene chloride layer no longer changes colour. Carry out a blank titration on a mixture of 10.0 mL of the freshly prepared 50 g/L solution of potassium iodide R, 20 mL of water R and 40 mL of hydrochloric acid R.
1 mL of 0.05 M potassium iodate is equivalent to mg of benzalkonium chloride where x is the average relative molecular mass of the sample.
LABELLING
The label states the content of ethanol (96 per cent), if any.
IMPURITIES
Specified impurities A, B, C.
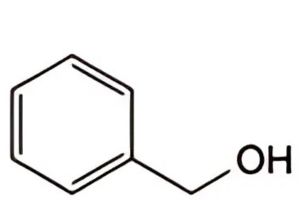
A. benzyl alcohol,
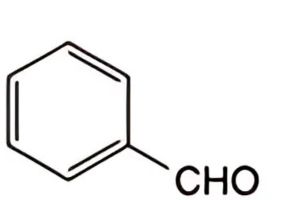
B. benzaldehyde,
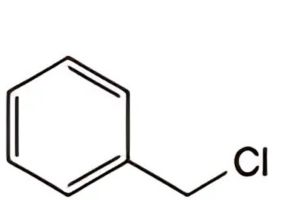
C. (chloromethyl)benzene.






