Action and use
Flavour.
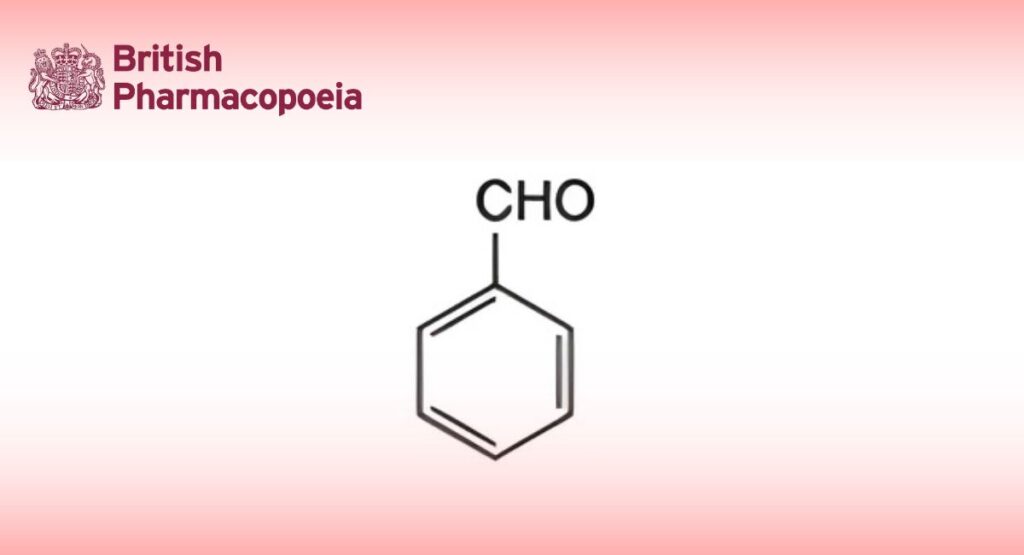
DEFINITION
Benzaldehyde contains not less than 98.0% w/w and not more than 100.5% w/w of C7H6O.
CHARACTERISTICS
A clear, colourless liquid.
Slightly soluble in water; miscible with ethanol (96%) and with ether.
TESTS
Refractive index
1.544 to 1.546, Appendix V E.
Weight per mL
1.043 to 1.049 g, Appendix V G.
Free acid
Not more than 1.0% w/v, calculated as benzoic acid, C7H6O2, when determined by the following method. To 10 mL add 20 mL of ethanol (96%) previously neutralised to phenolphthalein solution R1 and titrate with 0.1M sodium hydroxide VS using phenolphthalein solution R1 as indicator. Each mL of 0.1M sodium hydroxide VS is equivalent to 12.21 mg of C7H6O2.
Chlorinated compounds
Not more than 0.05% w/v, calculated as Cl, when determined by the following method. To 5 mL add 50 mL of isoamyl alcohol and 3 g of sodium and boil under a reflux condenser for 1 hour. Cool, add 50 mL of water and 15 mL of nitric acid, cool, add 5 mL of 0.1M silver nitrate VS, shake and titrate the excess silver nitrate with 0.1M ammonium thiocyanate VS using ammonium iron(III) sulfate solution R2 as indicator. Repeat the procedure without the substance being examined. The difference between the titrations represents the amount of silver nitrate required. Each mL of 0.1M silver nitrate VS is
equivalent to 3.545 mg of Cl.
ASSAY
Carry out the method for determination of aldehydes, Appendix X K, using 0.5 g. Each mL of 0.5M potassium hydroxide in ethanol (60%) VS is equivalent to 53.06 mg of C7H6O.
STORAGE
Benzaldehyde should be kept in a well-filled container, protected from light and stored at a temperature not exceeding 15°.