(Ph. Eur. monograph 1172)
C22H24FN3O2 381.4 2062-84-2
Action and use
Dopamine receptor antagonist; neuroleptic.
DEFINITION
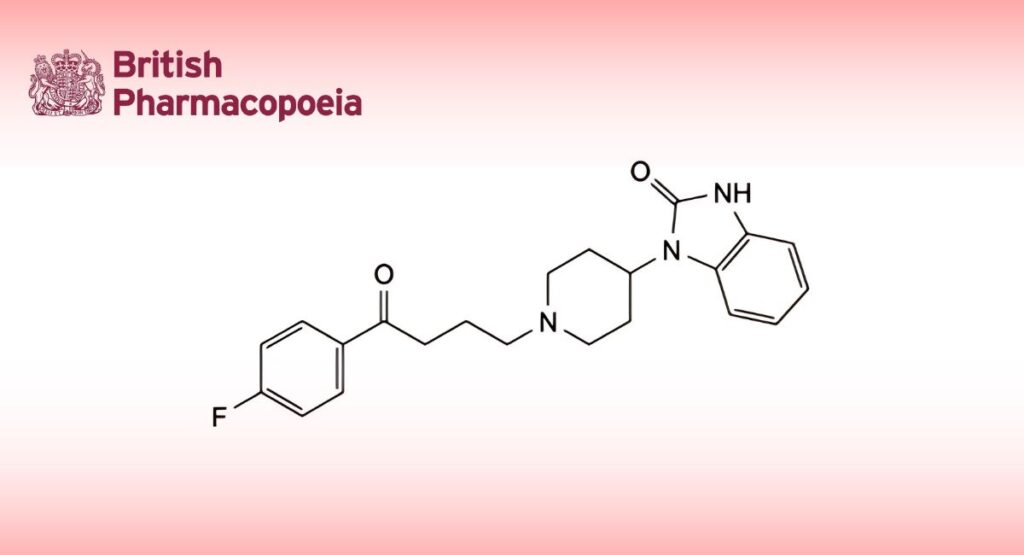
1-[1-[4-(4-Fluorophenyl)-4-oxobutyl]piperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Practically insoluble in water, freely soluble in dimethylformamide, soluble in methylene chloride, slightly soluble in ethanol (96 per cent).
It shows polymorphism (5.9).
IDENTIFICATION
First identification: A.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison benperidol CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in the minimum volume of methyl isobutyl ketone R, evaporate to dryness and record new spectra using the residues.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 30 mg of the substance to be examined in the mobile phase and dilute to 10 mL with the mobile phase.
Reference solution (a): Dissolve 30 mg of benperidol CRS in the mobile phase and dilute to 10 mL with the mobile phase.
Reference solution (b): Dissolve 30 mg of benperidol CRS and 30 mg of droperidol CRS in the mobile phase and dilute to 10 mL with the mobile phase.
Plate: TLC silica gel F254 plate R.
Mobile phase acetone R, methanol R (10:90 V/V).
Application: 10 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve about 10 mg in 5 mL of anhydrous ethanol R. Add 0.5 mL of dinitrobenzene solution R and 0.5 mL of 2 M alcoholic potassium hydroxide R. A violet colour is produced which becomes brownish-red after 20 min.
D. Mix about 5 mg with 45 mg of heavy magnesium oxide R and ignite in a crucible until an almost white residue is obtained (usually less than 5 min). Allow to cool, add 1 mL of water R, 0.05 mL of phenolphthalein solution R1 and about 1 mL of dilute hydrochloric acid R to render the solution colourless. Filter. To a freshly prepared mixture of 0.1 mL of alizarin S solution R and 0.1 mL of zirconyl nitrate solution R, add 1.0 mL of the filtrate. Mix, allow to stand for 5 min and compare the colour of the solution with that of a blank prepared in the same manner. The test solution is yellow and the blank is red.
TESTS
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution: Dissolve 0.10 g of the substance to be examined in dimethylformamide R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 2.5 mg of benperidol CRS and 2.5 mg of droperidol CRS in dimethylformamide R and dilute to 100.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with dimethylformamide R. Dilute 5.0 mL of this solution to 20.0 mL with dimethylformamide R.
Column:
— size: l = 0.1 m, Ø = 4.6 mm;
— stationary phase: base-deactivated octadecylsilyl silica gel for chromatography R (3 μm).
Mobile phase:
— mobile phase A: 10 g/L solution of tetrabutylammonium hydrogen sulfate R;
— mobile phase B: acetonitrile R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 15 | 100 → 60 | 0 → 40 |
| 15 – 20 | 60 | 40 |
| 20 – 25 | 100 | 0 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 275 nm.
Injection: 10 μL.
Relative retention: With reference to benperidol (retention time = about 6.5 min): impurity A = about 0.2;
impurity B = about 0.9; droperidol = about 1.1; impurity D = about 1.2; impurity E = about 1.3; impurity C = about 1.5.
System suitability: Reference solution (a):
— resolution: minimum 2.0 between the peaks due to benperidol and droperidol.
Limits:
— impurities A, B, C, D, E: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.25 per cent);
— unspecified impurities: for each impurity, not more than 0.4 times the area of the principal peak in the
chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— disregard limit: 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g in a platinum crucible.
ASSAY
Dissolve 0.300 g in 50 mL of a mixture of 1 volume of anhydrous acetic acid R and 7 volumes of methyl ethyl ketone R. Titrate with 0.1 M perchloric acid, using 0.2 mL of naphtholbenzein solution R as indicator.
1 mL of 0.1 M perchloric acid is equivalent to 38.14 mg of C22H24FN3O2.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D, E.
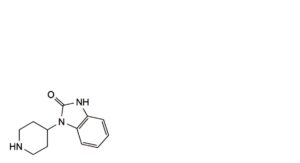
A. 1-(piperidin-4-yl)-1,3-dihydro-2H-benzimidazol-2-one,
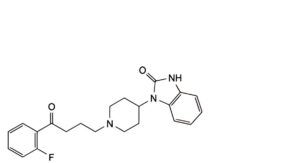
B. 1-[1-[4-(2-fluorophenyl)-4-oxobutyl]piperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one,
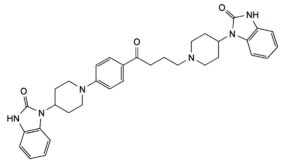
C. 1-[1-[4-oxo-4-[4-[4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl]phenyl]butyl]piperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one,
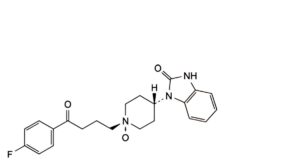
D. cis-1-[1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl 1-oxide]-1,3-dihydro-2H-benzimidazol-2-one,
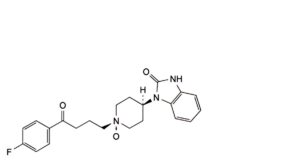
E. trans-1-[1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl 1-oxide]-1,3-dihydro-2H-benzimidazol-2-one.