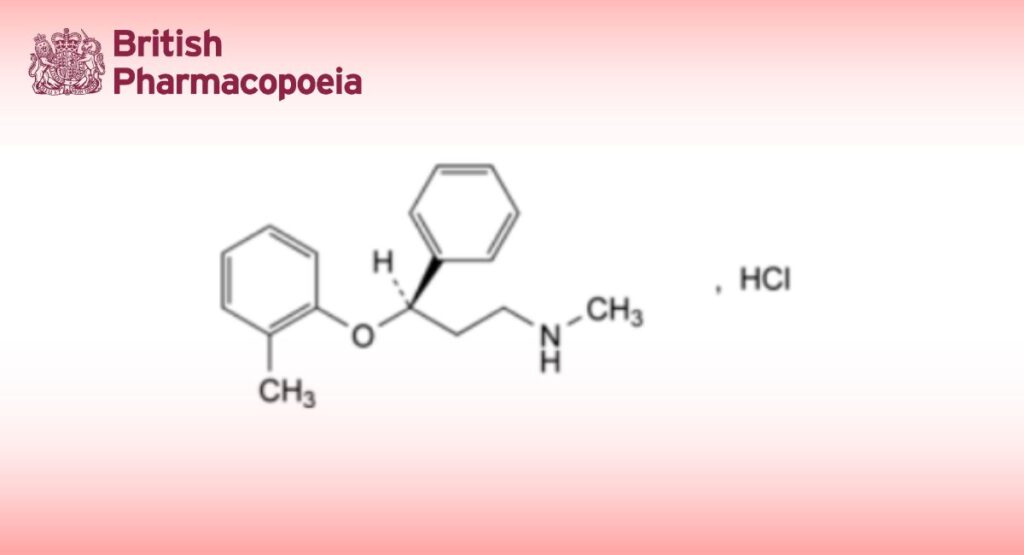
(Ph. Eur. monograph 2640)
C17H22ClNO 291.8 82248-59-7
Action and use
Noradrenaline reuptake inhibitor; treatment of attention deficit hyperactivity disorder (ADHD).
DEFINITION
(3R)-N-Methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine hydrochloride.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Sparingly soluble in water, soluble in anhydrous ethanol, practically insoluble in heptane.
It shows polymorphism (5.9).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison atomoxetine hydrochloride CRS.
Atomoxetine Hydrochloride
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in anhydrous ethanol R, evaporate to dryness and record new spectra using the residues.
B. Isomeric purity (see Tests).
C. It gives reaction (a) of chlorides (2.3.1).
TESTS
Isomeric purity
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution: Dissolve 35.0 mg of the substance to be examined in 2.5 mL of anhydrous ethanol R, sonicate until dissolution is complete and dilute to 10.0 mL with heptane R.
Reference solution (a): Dissolve 3.5 mg of atomoxetine impurity B CRS and 1 mg of atomoxetine impurity D CRS in 5 mL of anhydrous ethanol R, sonicate until dissolution is complete and dilute to 20.0 mL with heptane R.
Reference solution (b): Dissolve 35.0 mg of the substance to be examined in 2.5 mL of anhydrous ethanol R. Add 1.0 mL of reference solution (a) and dilute to 10.0 mL with heptane R.
Reference solution (c): Dilute 1.0 mL of reference solution (a) to 100.0 mL with heptane R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: cellulose derivative of silica gel for chiral separation R (5 μm).
Mobile phase: Mix 1.5 mL of diethylamine R, 2.0 mL of trifluoroacetic acid R and 150.0 mL of 2-propanol R and dilute to 1000 mL with heptane R.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 273 nm.
Injection: 10 μL of the test solution and reference solutions (b) and (c).
Run time: 1.3 times the retention time of atomoxetine.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities B and D.
Relative retention: With reference to atomoxetine (retention time = about 12 min): impurity B = about 0.5;
impurity D = about 0.6. System suitability Reference solution (b):
— resolution: minimum 1.8 between the peaks due to impurities B and D.
Limits:
— impurity B: maximum 0.5 per cent;
— impurity D: maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— disregard limit: the area of the peak due to impurity B in the chromatogram obtained with reference solution (c) (0.05 per cent); disregard any peak with a relative retention with reference to atomoxetine of about 0.7 (impurity A).
Related substances
Liquid chromatography (2.2.29).
Solution A: Dissolve 5.9 g of sodium octanesulfonate monohydrate R in 1000 mL of a 2.9 g/L solution of phosphoric acid R previously adjusted to pH 2.5 with a 280 g/L solution of potassium hydroxide R.
Test solution (a): Dissolve 25 mg of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Test solution (b): Dissolve 25.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of test solution (a) to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 7.5 mg of 3-(methylamino)-1-phenylpropan-1-ol R (impurity H) and 5 mg of mandelic acid R (impurity E) in test solution (b) and dilute to 50 mL with test solution (b).
Reference solution (c): Dissolve 5 mg of atomoxetine for impurity A identification CRS in the mobile phase and dilute to 20 mL with the mobile phase.
Reference solution (d): Dissolve 25.0 mg of atomoxetine hydrochloride CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography R (3.5 μm);
— temperature: 40 °C.
Mobile phase propanol R, solution A (27:73 V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 10 μL of test solution (a) and reference solutions (a), (b) and (c).
Run time: 2.5 times the retention time of atomoxetine.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities E and H; use the chromatogram supplied with atomoxetine for impurity A identification CRS and the chromatogram obtained with reference solution (c) to identify the peak due to impurity A.
Relative retention With reference to atomoxetine (retention time = about 10 min): impurity E = about 0.2;
impurity H = about 0.3; impurity A = about 0.7.
System suitability Reference solution (b):
— resolution: minimum 5.0 between the peaks due to impurities E and H.
Calculation of percentage contents:
— for each impurity, use the concentration of atomoxetine hydrochloride in reference solution (a).
Limits:
— impurity A: maximum 0.3 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at 105 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution (b) and reference solution (d).
Calculate the percentage content of C17H22ClNO taking into account the assigned content of atomoxetine hydrochloride CRS.
IMPURITIES
Specified impurities A, B, D.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).
It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) C, E, F, G, H.
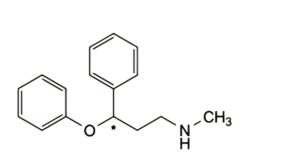
A. N-methyl-3-phenoxy-3-phenylpropan-1-amine,
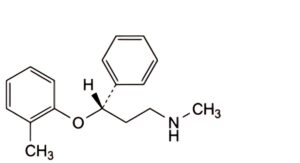
B. (3S)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine,
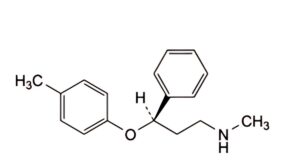
C. (3R)-N-methyl-3-(4-methylphenoxy)-3-phenylpropan-1-amine,
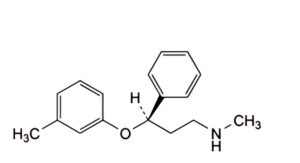
D. (3R)-N-methyl-3-(3-methylphenoxy)-3-phenylpropan-1-amine,
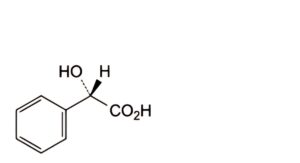
E. (2S)-2-hydroxy-2-phenylacetic acid (L-mandelic acid),
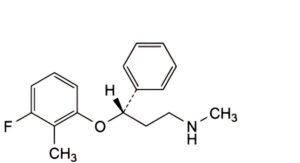
F. (3S)-3-(3-fluoro-2-methylphenoxy)-N-methyl-3-phenylpropan-1-amine,
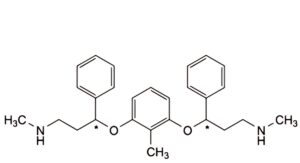
G. 3,3′-[(2-methylbenzene-1,3-diyl)bis(oxy)]bis(N-methyl-3-phenylpropan-1-amine),
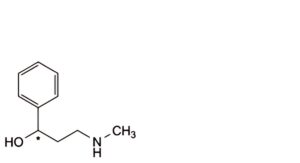
H. 3-(methylamino)-1-phenylpropan-1-ol.