(Ph. Eur. monograph 1688)
C13H21ClN2O3S 320.8 23964-57-0
Action and use
Local anaesthetic.
DEFINITION
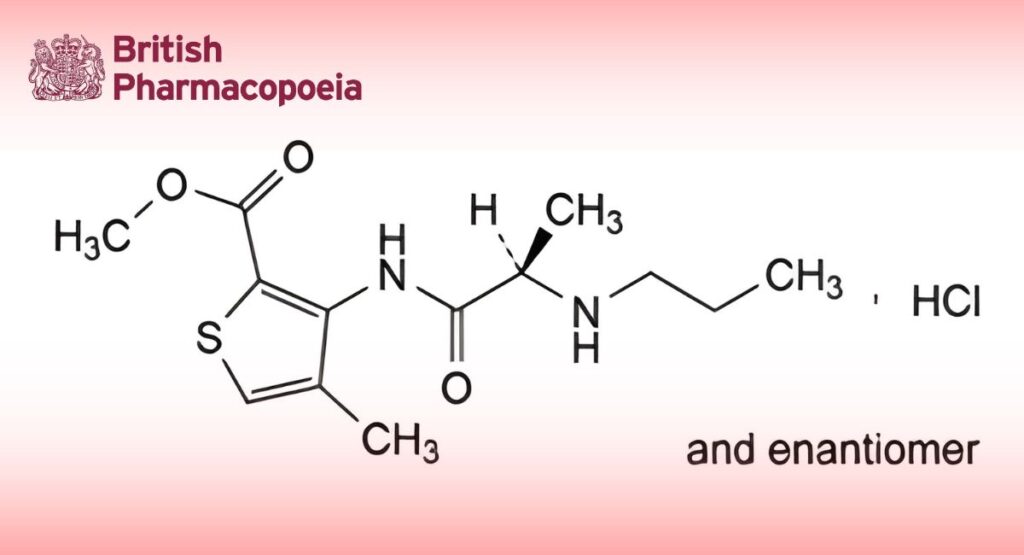
Methyl 4-methyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxylate hydrochloride.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water and in ethanol (96 per cent).
IDENTIFICATION
First identification: B, D.
Second identification: A, C, D.
A. Dissolve 50.0 mg in a 1 g/L solution of hydrochloric acid R and dilute to 100.0 mL with the same acid. Dilute 5.0 mL of the solution to 100.0 mL with a 1 g/L solution of hydrochloric acid R. Examined between 200 nm and 350 nm (2.2.25), the solution shows an absorption maximum at 272 nm. The specific absorbance at the maximum is 290 to 320.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation: Place dropwise 20 μL of the test solution on 300 mg discs.
Test solution: Dissolve 0.1 g in 5 mL of water R, add 3 mL of a saturated solution of sodium hydrogen carbonate R and shake twice with 2 mL of methylene chloride R. Combine the methylene chloride layers, dilute to 5.0 mL with methylene chloride R and dry over anhydrous sodium sulfate R.
Comparison: articaine hydrochloride CRS.
C. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 20 mg of the substance to be examined in 5 mL of ethanol (96 per cent) R.
Reference solution: Dissolve 20 mg of articaine hydrochloride CRS in 5 mL of ethanol (96 per cent) R.
Plate: TLC silica gel F254 plate R.
Mobile phase: triethylamine R, ethyl acetate R, heptane R (10:35:65 V/V/V).
Application: 5 μL.
Development: Over a path of 15 cm.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
D. It gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 0.50 g in water R and dilute to 10 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution BY6 (2.2.2, Method I).
pH (2.2.3)
4.2 to 5.2.
Dissolve 0.20 g in carbon dioxide-free water R and dilute to 20.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 10.0 mg of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 5.0 mg of articaine impurity A CRS and 2.5 mg of articaine impurity E CRS in the mobile phase and dilute to 50.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 50.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 45 °C.
Mobile phase: Mix 25 volumes of acetonitrile R and 75 volumes of a solution prepared as follows: dissolve 2.02 g of sodium heptanesulfonate R and 4.08 g of potassium dihydrogen phosphate R in water R and dilute to 1000 mL with the same solvent. Adjust to pH 2.0 with phosphoric acid R.
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 276 nm.
Injection: 10 μL.
Run time: 5 times the retention time of articaine.
Relative retention: With reference to articaine (retention time = about 9 min): impurity A = about 0.8; impurity E = about 0.86.
System suitability: Reference solution (b):
— resolution: minimum 1.2 between the peaks due to impurities A and E.
Limits:
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— sum of impurities other than A: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 5 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in a mixture of 5.0 mL of 0.01 M hydrochloric acid and 50 mL of ethanol (96 per cent) R. Carry out a potentiometric titration (2.2.20) using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 32.08 mg of C13H21ClN2O3S.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, D, E, F, G, H, I, J.
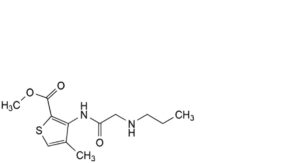
A. methyl 4-methyl-3-[[2-(propylamino)acetyl]amino]thiophene-2-carboxylate (acetamidoarticaine),
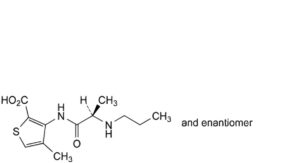
B. 4-methyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxylic acid (articaine acid),
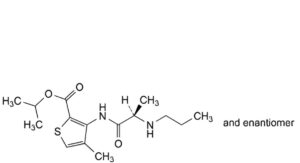
C. 1-methylethyl 4-methyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxylate (articaine isopropyl ester),
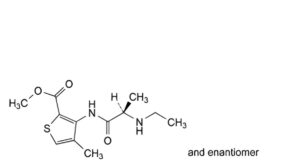
D. methyl 3-[[(2RS)-2-(ethylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate (ethylarticaine),
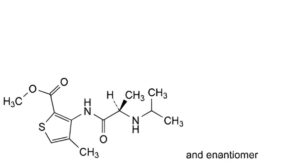
E. methyl 4-methyl-3-[[(2RS)-2-[(1-methylethyl)amino]propanoyl]amino]thiophene-2-carboxylate (isopropylarticaine),
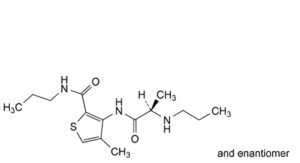
F. 4-methyl-N-propyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxamide (articaine acid propionamide),
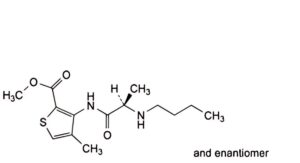
G. methyl 3-[[(2RS)-2-(butylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate (butylarticaine),
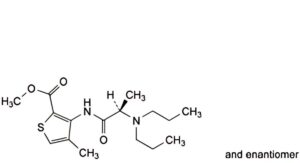
H. methyl 3-[[(2RS)-2-(dipropylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate (dipropylarticaine),
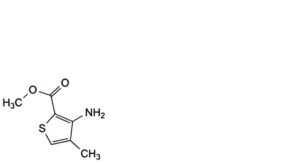
I. methyl 3-amino-4-methylthiophene-2-carboxylate (3-aminoarticaine),
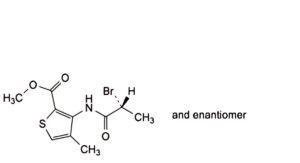
J. methyl 3-[[(2RS)-2-bromopropanoyl]amino]-4-methylthiophene-2-carboxylate (bromo compound).