(Ph. Eur. monograph 0580)
C284H432N84O79S7 6511 9087-70-1
Action and use
Antifibrinolytic.
DEFINITION
Aprotinin is a polypeptide consisting of a chain of 58 amino acids. It inhibits stoichiometrically the activity of several proteolytic enzymes such as chymotrypsin, kallikrein, plasmin and trypsin. It contains not less than 3.0 Ph. Eur. U. of aprotinin activity per milligram, calculated with reference to the dried substance.
PRODUCTION
The animals from which aprotinin is derived must fulfil the requirements for the health of animals suitable for human consumption.
The method of manufacture is validated to demonstrate that the product, if tested, would comply with the following test.
Histamine (2.6.10)
Maximum 0.2 μg of histamine base per 3 Ph. Eur. U.
CHARACTERS
Appearance
Almost white hygroscopic powder.
Solubility
Soluble in water and in isotonic solutions, practically insoluble in organic solvents.
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution: Solution S (see Tests).
Reference solution: Dilute aprotinin solution BRP in water R to obtain a concentration of 15 Ph. Eur. U./mL.
Plate: TLC silica gel G plate R.
Mobile phase water R, glacial acetic acid R (80:100 V/V) containing 100 g/L of sodium acetate R.
Application: 10 μL.
Development: Over a path of 12 cm.
Drying: In air.
Detection: Spray with a solution of 0.1 g of ninhydrin R in a mixture of 6 mL of a 10 g/L solution of cupric chloride R, 21 mL of glacial acetic acid R and 70 mL of anhydrous ethanol R. Dry the plate at 60 °C.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
B. Determine the ability of the substance to be examined to inhibit trypsin activity using the method described below.
Test solution: Dilute 1 mL of solution S to 50 mL with buffer solution pH 7.2 R.
Trypsin solution: Dissolve 10 mg of trypsin for aprotinin assay BRP in 0.002 M hydrochloric acid and dilute to 100 mL with the same acid.
Casein solution: Dissolve 0.2 g of casein R in buffer solution pH 7.2 R and dilute to 100 mL with the same buffer solution.
Precipitating: solution glacial acetic acid R, water R, anhydrous ethanol R (1:49:50 V/V/V).
Mix 1 mL of the test solution with 1 mL of the trypsin solution. Allow to stand for 10 min and add 1 mL of the casein solution. Incubate at 35 °C for 30 min. Cool in iced water and add 0.5 mL of the precipitating solution. Shake and allow to stand at room temperature for 15 min. The solution is cloudy. Carry out a blank test under the same conditions using buffer solution pH 7.2 R instead of the test solution. The solution is not cloudy.
TESTS
Solution S
Prepare a solution of the substance to be examined containing 15 Ph. Eur. U./mL, calculated from the activity stated on the label.
Appearance of solution
Solution S is clear (2.2.1).
Absorbance (2.2.25)
Maximum 0.80 by measuring at the absorption maximum at 277 nm.
Prepare a solution of the substance to be examined containing 3.0 Ph. Eur. U./mL.
Des-Ala-aprotinin and des-Ala-des-Gly-aprotinin
Capillary zone electrophoresis (2.2.47): use the normalisation procedure.
Test solution: Prepare a solution of the substance to be examined in water R containing not less than 1 Ph. Eur. U./mL.
Reference solution: Dilute aprotinin solution BRP in water R to obtain the same concentration as the test solution.
Capillary:
— material: uncoated fused silica;
— size: effective length = 45-60 cm, Ø = 75 μm.
Temperature 25 °C.
CZE buffer: Dissolve 8.21 g of potassium dihydrogen phosphate R in 400 mL of water R, adjust to pH 3.0 with phosphoric acid R, dilute to 500.0 mL with water R and filter through a membrane filter (nominal pore size 0.45 μm).
Detection: Spectrophotometer at 214 nm.
Between-run rinsing: Rinse the capillary for at least 1 min with 0.1 M sodium hydroxide filtered through a membrane filter (nominal pore size 0.45 μm) and for 2 min with the CZE buffer.
Injection: Under pressure or vacuum (for example, 3 s at a differential pressure of 3.5 kPa).
Migration: Apply a field strength of 0.2 kV/cm, using the CZE buffer as the electrolyte in both buffer reservoirs.
Run time: 30 min.
Identification of impurities: Use the electropherogram supplied with aprotinin solution BRP and the electropherogram obtained with the reference solution to identify the peaks due to impurities A and B.
Relative migration: With reference to aprotinin (migration time = about 22 min): impurity A = about 0.98;
impurity B = about 0.99.
System suitability Reference solution after at least 6 injections:
— migration time: aprotinin = 19.0 min to 25.0 min;
— resolution: minimum 0.8 between the peaks due to impurities A and B; minimum 0.5 between the peaks due timpurity B and aprotinin;
— peak distribution: the electropherogram obtained is qualitatively and quantitatively similar to the electropherogram supplied with aprotinin solution BRP;
— height of the principal peak: at least 1000 times the height of the baseline noise. If necessary, adjust the sample load to give peaks of sufficient height.
Limits:
— impurity A: maximum 8.0 per cent;
— impurity B: maximum 7.5 per cent.
Pyroglutamyl-aprotinin and related compounds
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution: Prepare a solution of the substance to be examined in mobile phase A, containing about 5 Ph. Eur. U./mL.
Reference solution: Dissolve the contents of a vial of aprotinin for system suitability CRS in 2.0 mL of mobile phase A.
Column:
— size: l = 0.075 m, Ø = 7.5 mm;
— stationary phase: strong cation-exchange silica gel for chromatography R (10 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: dissolve 3.52 g of potassium dihydrogen phosphate R and 7.26 g of disodium hydrogen phosphate dihydrate R in 1000 mL of water for chromatography R; filter and degas;
— mobile phase B: dissolve 3.52 g of potassium dihydrogen phosphate R, 7.26 g of disodium hydrogen phosphate dihydrate R and 66.07 g of ammonium sulfate R in 1000 mL of water for chromatography R; filter and degas;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 21 | 92 → 64 | 8 → 36 |
| 21 – 30 | 64 → 0 | 36 → 100 |
Flow rate 1.0 mL/min.
Detection: Spectrophotometer at 210 nm.
Injection: 40 μL.
Relative retention: With reference to aprotinin (retention time = 17.0 min to 20.0 min): impurity C = about 0.9.
System suitability: Reference solution:
— resolution: minimum 1.5 between the peaks due to impurity C and aprotinin;
— symmetry factor: maximum 1.3 for the peak due to aprotinin.
Limits:
— impurity C: maximum 1.0 per cent;
— any other impurity: maximum 0.5 per cent;
— sum of impurities other than C: maximum 1.0 per cent.
Aprotinin oligomers
Size-exclusion chromatography (2.2.30): use the normalisation procedure.
Test solution: Prepare a solution of the substance to be examined in water R containing about 5 Ph. Eur. U./mL.
Reference solution: Treat the substance to be examined to obtain about 2 per cent aprotinin oligomers. For example, heat freeze-dried aprotinin at about 110 °C for about 4 h. Then dissolve in water R to obtain a concentration of about 5 Ph. Eur. U./mL.
Column 3 columns coupled in series:
— size: l = 0.30 m, Ø = 7.8 mm;
— stationary phase: hydrophilic silica gel for chromatography R of a grade suitable for fractionation of globular
proteins in the relative molecular mass range of 20 000 to 10 000 000 (8 μm).
Mobile phase acetonitrile R, glacial acetic acid R, water for chromatography R (2:2:6 V/V/V); filter and degas.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 277 nm.
Injection: 100 μL.
Run time: 40 min.
Relative retention: With reference to aprotinin monomer (retention time = 24.5 min to 25.5 min): aprotinin
dimer = about 0.9.
System suitability: Reference solution:
— resolution: minimum 1.3 between the peaks due to aprotinin dimer and monomer;
— symmetry factor: maximum 2.5 for the peak due to aprotinin monomer.
Limit:
— total: maximum 1.0 per cent.
Loss on drying (2.2.32)
Maximum 6.0 per cent, determined on 0.100 g by drying in vacuo.
Bacterial endotoxins (2.6.14)
Less than 0.14 IU per European Pharmacopoeia Unit of aprotinin, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
ASSAY
The activity of aprotinin is determined by measuring its inhibitory action on a solution of trypsin of known activity. The inhibiting activity of the aprotinin is calculated from the difference between the initial activity and the residual activity of the trypsin.
The inhibiting activity of aprotinin is expressed in European Pharmacopoeia Units. 1 Ph. Eur. U. inhibits 50 per cent of the enzymatic activity of 2 microkatals of trypsin.
Use a reaction vessel with a capacity of about 30 mL, provided with:
— a device that will maintain a temperature of 25 ± 0.1 °C;
— a stirring device, such as a magnetic stirrer;
— a lid with 5 holes for accommodating the electrodes, the tip of a burette, a tube for the admission of nitrogen and the introduction of the reagents.
An automatic or manual titration apparatus may be used. In the latter case the burette is graduated in 0.05 mL and the pH- meter is provided with a wide reading scale and glass-silver-silver chloride or other suitable electrodes.
Test solution: Prepare a solution of the substance to be examined in 0.0015 M borate buffer solution pH 8.0 R expected to contain 1.67 Ph. Eur. U./mL (about 0.6 mg (m mg) per millilitre).
Trypsin solution: Prepare a solution of trypsin for aprotinin assay BRP containing about 0.8 microkatals per millilitre, using 0.001 M hydrochloric acid as the solvent. Use a freshly prepared solution and keep in iced water.
Trypsin and aprotinin solution: To 4.0 mL of the trypsin solution add 1.0 mL of the test solution. Dilute immediately to 40.0 mL with 0.0015 M borate buffer solution pH 8.0 R. Allow to stand at room temperature for 10 min and then keep in iced water. Use within 6 h of preparation.
Dilute trypsin solution: Dilute 0.5 mL of the trypsin solution to 10.0 mL with 0.0015 M borate buffer solution pH 8.0 R. Allow to stand at room temperature for 10 min and then keep in iced water.
Maintain an atmosphere of nitrogen in the reaction flask and stir continuously; introduce 9.0 mL of 0.0015 M borate buffer solution pH 8.0 R and 1.0 mL of a freshly prepared 6.9 g/L solution of benzoylarginine ethyl ester hydrochloride R. Adjust to pH 8.0 with 0.1 M sodium hydroxide. When the temperature has reached equilibrium at 25 ± 0.1 °C, add 1.0 mL of the trypsin and aprotinin solution and start a timer. Maintain at pH 8.0 by the addition of 0.1 M sodium hydroxide and note the volume added every 30 s. Continue the reaction for 6 min. Determine the number of millilitres of 0.1 M sodium hydroxide used per second (n1 mL). Carry out, under the same conditions, a titration using 1.0 mL of the dilute trypsin solution. Determine the number of millilitres of 0.1 M sodium hydroxide used per second (n2 mL).
Calculate the aprotinin activity in European Pharmacopoeia Units per milligram using the following expression:
4000(2n2-n1)/m
The estimated activity is not less than 90 per cent and not more than 110 per cent of the activity stated on the label.
STORAGE
In an airtight, tamper-evident container, protected from light.
LABELLING
The label states:
— the number of European Pharmacopoeia Units of aprotinin activity per milligram;
— where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
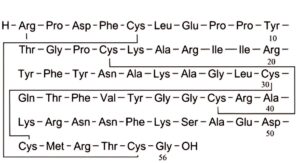
A. aprotinin-(1-56)-peptide,
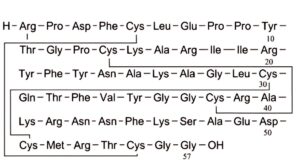
B. aprotinin-(1-57)-peptide,
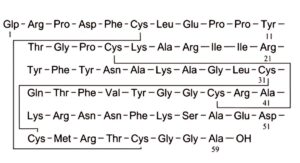
C. (5-oxoprolyl)aprotinin (pyroglutamylaprotinin).






