(Ph. Eur. general texts 5.2.5)
1 SCOPE
Materials which are used during the manufacture of immunological veterinary medicinal products (IVMPs) may be susceptible to contamination by extraneous agents (bacteria, mycoplasma, fungi and viruses). This general chapter deals exclusively with the issue of living replicative extraneous agents, and therefore throughout the chapter, the term ‘extraneous agents’ is to be understood as referring to living replicative organisms.
The requirements set out in this general chapter apply to materials used at all stages of manufacture, in order to ensure that IVMPs are free of extraneous agents.
Throughout this general chapter, the term ‘materials’ is to be understood as referring to starting materials of animal or human origin. This includes seed materials, substrates for production (e.g. embryonated eggs, cell substrates and animals), ingredients in culture media and substances produced on a batch basis that are used in the vaccine production process (e.g. excipients or adjuvants).
2 GENERAL PRINCIPLES AND REQUIREMENTS
Materials of animal or human origin comply with the requirements of the European Pharmacopoeia (where a relevant monograph exists).
Restrictions are placed on the use of materials of animal or human origin because of safety concerns associated with the pathogens they may contain. The following general actions are recommended to prevent contamination throughout the entire production process and in the final product:
— wherever practicable, avoid or keep to a minimum the use of substances of animal or human origin;
— as far as practically possible, use materials expected or demonstrated to be free from extraneous agents;
— use standardised and well-controlled production processes taking into account quality systems in place, in order to prevent the introduction of extraneous agents during production;
— apply processing treatments that remove or inactivate extraneous agents.
According to the principles of risk management, as detailed below, the list of extraneous agents to be tested in the final product is limited to those that cannot be excluded by other means.
General requirements:
— any master seed lot (after processing, if relevant) found to contain extraneous agents of any kind, other than the species and strain stated, is unsuitable for vaccine production;
— any substrate (after processing, if relevant) found to contain any extraneous agent shall be discarded or used only in exceptional and justified circumstances;
— any batch of substance (after inactivation or processing, if relevant) found to contain any extraneous agents shall be discarded or used only in exceptional and justified circumstances; to be accepted for use, further processing must be carried out that will ensure elimination or inactivation of the extraneous agent in the final product, and it shall then be demonstrated that the elimination or inactivation has been satisfactory;
— unless otherwise prescribed, any final product found to contain any extraneous agent shall be discarded.
3 RISK MANAGEMENT
No single measure or combination of measures can ensure the safety of use of materials of animal and human origin, but they can reduce the risks associated with such use. It is therefore necessary for manufacturers of IVMPs to take this into account when choosing a material for use in product manufacture, and to conduct a risk assessment taking into account the origin and nature of the material and the manufacturing steps to which it is subjected.
In addition, risk management procedures must be applied, and any residual risk must be evaluated in relation to the potential benefits derived from the use of the material to manufacture the IVMP.
3-1 RISK ASSESSMENT
The risk of contamination of the materials and the resulting IVMP with extraneous agents must be assessed.
The risk assessment must take into account:
— animal diseases occurring in the region or country of origin of the animals from which the material is obtained;
— potential infectious diseases that may occur in the source species;
— potential infectious diseases that may occur in the target species;
— likely infectivity in the source organ or tissue, and results of any test for extraneous agents already available;
— the effectiveness of any specific inactivation treatment applied to materials, which depends on the resistance of the potential extraneous agent to this treatment and the extent of contamination.
From this information, and based on the lists of extraneous agents provided in Annex I, a list of the extraneous agents which may be present in the material is considered as part of the risk assessment. The lists provided in Annex I do not preclude additional agents from being considered, if necessary.
When assessing the risk of these extraneous agents being present in the final product, the following must be considered:
— the stage of production at which contamination may occur and all subsequent steps in the manufacturing process;
— the capacity of the production process to amplify an extraneous agent (e.g. a viral contaminant may multiply during production on a cellular substrate, but not on an acellular medium; viruses unable to multiply in vitro) or to remove it (purification, inactivation, etc.).
The risk assessment may have to be repeated and the risk management steps described below re-evaluated and revised in order to take account of any changes and to ensure that the material always complies with these requirements, for example:
— changes in the incidence of diseases occurring in the region or country of origin of the animals used as the source of the material, including emerging diseases (new pathogens);
— any changes made to the production process or source materials.
3-2 RISK CONTROL
The risk assessment allows appropriate control measures to be defined and applied at all stages, from the sourcing of materials to the final product stage, in order to ensure the absence of risk of extraneous agents.
Depending on the material, one or a combination of the following measures is applied:
— placing restrictions on the source of the material and auditing these restrictions;
— using validated inactivation procedures;
— demonstrating the ability of a production step to remove or inactivate extraneous agents;
— testing for extraneous agents in cases where their presence cannot be excluded during the risk assessment.
The need to test the final product for the presence of viral extraneous agents and the testing strategy must be evaluated on the basis of a risk assessment as described in section 3-1
For viral vaccines, testing for viral extraneous agents in the final product may not be necessary, provided all of the following general conditions are met:
— the vaccines are produced according to well-established quality systems (e.g. under conditions of good manufacturing practice);
— the master seed is free from extraneous agents (based either on risk assessment or testing);
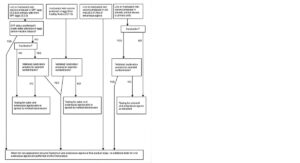
— the other materials used in the production process are free from extraneous agents (based on risk assessment, testing or treatment); amongst these materials, the quality of the substrates can be considered according to a decision tree of the type proposed in Annex II to possibly alleviate testing of extraneous agents in final products.
4 CONTROL MEASURES
4-1 CONTROL MEASURES FOR STARTING MATERIALS
4-1-1 Preventive measures during sourcing and preparation
4-1-1-1 Seed lots
A record of the origin, date of isolation and passage history (including purification and characterisation procedures) is maintained for each master seed lot.
Whenever possible, restrictions are placed on substrates and substances used for the propagation of the viruses or bacteria between the initial isolate and the established master seed (i.e. the use of embryonated eggs free from specified pathogens (SPF) for isolation) to prevent the introduction of extraneous agents into the seed material. Such measures are documented and taken into account in the risk assessment.
4-1-1-2 Substrates for production
When the master seed lot and all subsequent passages are propagated on cells, embryonated eggs or in animals, such substrates have been shown to be suitable for vaccine production.
4-1-1-2-1 Cell substrates
Cell cultures used in the production of vaccines for veterinary use comply with the requirements of general chapter 5.2.4.
4-1-1-2-2 Embryonated hens’ eggs
Where a monograph refers to SPF chicken flocks, these flocks comply with the requirements of general chapter 5.2.2.
Where vaccine organisms are grown in chicken embryos for the production of a master seed lot and for all passages of a microorganism up to and including the working seed lot, eggs from SPF flocks (5.2.2) are used.
For production of inactivated vaccines, where vaccine organisms are grown in chicken embryos from the working seed lot onwards, such embryos are derived either from SPF flocks (5.2.2) or from healthy non-SPF flocks (5.2.13).
For production of live vaccines, where vaccine organisms are grown in chicken embryos from the working seed lot onwards, such embryos are derived from SPF flocks (5.2.2).
4-1-1-2-3 Animals
When animals are used for the production of immunosera, they comply with the requirements of the general monograph Immunosera for veterinary use (0030). Where the use of animals or animal tissues in the production of the vaccines is unavoidable, the following requirements apply.
Chickens used for the production of vaccines are obtained from an SPF flock (5.2.2).
Other animals used for the production of vaccines are free from specified pathogens.
The animals used are exclusively reserved for production of vaccines. They are maintained under conditions protecting them from exposure to disease. These animals, and any animals in contact with them, are tested and shown to be free from a set list of infectious agents, and they are re-tested at suitable intervals. The list of agents considered relevant for testing is established by risk assessment which in particular takes into account their species, the target species if different and the geographical area they have been sourced from. The feed is obtained from a controlled source. Where applicable for the species used, measures are taken to avoid contamination with agents of transmissible spongiform encephalopathies.
Any animals introduced into the herd are from a known source and have a known breeding and rearing history. The introduction of animals into the herd follows specified procedures, including defined quarantine measures. During the quarantine period, the animals are observed for clinical signs of diseases. At the end of the quarantine period, the animals are tested according to the outcome of a risk assessment. The risk assessment is conducted taking into account their species, the target species if different, the clinical signs of disease if any and the geographical area they have been
sourced from.
Any routine or therapeutic medicinal treatment administered to the animals either in quarantine or thereafter must be recorded.
4-1-1-3 Media for vaccine production
All documentation on media should be provided in the risk assessment, including a list of ingredients derived from animals and the inactivation procedure (such as sterilisation) used.
4-1-1-4 Substances of animal origin
All substances of animal origin used in the manufacture (including formulation) of IVMPs must be from a known and documented source (including species of origin, and region or country of origin of source animals and tissues).
Substances of animal origin are prepared from a homogeneous bulk designated with a batch number. A batch may contain substances derived from as many animals as desired but once defined and given a batch number, the batch is not added to or contaminated in any way.
Unless otherwise justified, the use of substances of animal or human origin as constituents in the formulation of IVMPs (i.e. excipients or adjuvants) is not acceptable except where such substances are considered free of extraneous agents by a risk assessment approach or are subject to a treatment validated for the inactivation or elimination of extraneous agents.
4-1-2 Treatment of starting materials to remove or inactivate extraneous agents
The production method used to prepare the material of animal origin may contribute to the removal or inactivation of extraneous agents. Additional treatments (e.g. inactivation) may be applied.
The inactivation procedure and/or other processing steps chosen shall have been validated and shown to be capable of reducing the titre of potential extraneous agents in the substance concerned by a cumulative factor of at least 10 .
If this reduction in titre cannot be shown experimentally, a maximum pre-treatment titre of the extraneous agent must be set, taking into account the reduction in titre afforded by the inactivation/processing step and including a safety margin factor of 100. Each batch of substance must be tested to determine the pre-treatment starting titre and to confirm that it is no greater than the specified limit, unless proper risk assessment based on suitable valid data shows that titres will always be at least 100 times lower than the titre that can be effectively inactivated.
The validation of the procedure(s) is conducted with a suitable representative range of viruses covering different sizes and types (enveloped and non-enveloped, DNA and RNA, single- and double-stranded), including test viruses with different degrees of resistance (e.g. temperature, pH), and taking into account the type of procedure(s) to be applied and the viruses that may be present in the material. The evidence for the efficacy of the procedure may take the form of references to published literature and/or experimental data generated by the manufacturer, but must be relevant to the conditions that will be present during the production and inactivation/processing of the material.
4-2 CONTROL MEASURES DURING PRODUCTION
4-2-1 Preventive measures
Unless otherwise justified and authorised, cells and viruses/bacteria/parasites for vaccine production are handled according to a seed lot system.
Cross contamination is avoided during production by the application of well-established quality systems (e.g. by production under conditions of good manufacturing practice).
4-2-2 Removal or inactivation of extraneous agents during production
During production, some processing steps can lead to removal or inactivation of possible contaminants.
For instance, in the case of inactivated IVMPs, the method used for inactivation of the active ingredient may be considered as a means of inactivating possible contaminants from materials of animal origin used in the manufacture of this active ingredient.
Likewise, for inactivated vaccines produced on embryonated eggs from healthy flocks, the inactivation process applied to the active ingredient may be considered as a means of inactivating potential contaminants.
4-3 METHODS OF DETECTION OF EXTRANEOUS AGENTS
4-3-1 General prerequisites
This section describes the general approach for methods to detect extraneous agents. As the materials under test vary substantially, it is not possible to describe all suitable methods and therefore any method that fulfils the requirements described in this general chapter may be used. The results of the tests are acceptable if the method has been demonstrated to provide adequate sensitivity and specificity for the detection of the targeted extraneous agent.
Quality control samples, such as appropriate positive run controls with a specified content of a representative agent and negative run controls, are included in each test run to validate the results and evaluate test performance.
In respect of the principles of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, the European Pharmacopoeia Commission is committed to the reduction of animal usage wherever possible in pharmacopoeial testing, and encourages the use of alternative procedures.
Molecular methods such as specific nucleic acid amplification techniques (NAT) with broad detection capabilities may be used either as an alternative to in vivo tests or as a supplement/alternative to in vitro culture tests based on the risk assessment.
The results of molecular methods require appropriate interpretation and further investigation may be necessary. For example, if a positive signal from NAT detection methods is obtained, other in vitro methods are used to verify and document the absence of viability of possible contaminants.
In the case of divergent results produced by several different methods, a risk assessment must be performed. Exceptionally, in the absence of any available in vitro test method, the use of in vivo tests methods is regarded as acceptable provided the risk assessment justifies the need for the test.
4-3-2 Specific information
Sterility Testing for bacteria and fungi is performed in accordance with general chapter 2.6.1. For bacteria and fungi that are not detectable by the sterility test (e.g. intracellular pathogens), other suitable methods are used, e.g. NAT (2.6.21).
Mycoplasmas Testing for mycoplasmas is performed in accordance with general chapter 2.6.7.
Extraneous viruses Testing for extraneous viruses is performed using methods involving molecular techniques, e.g. NAT (2.6.21), or using culture methods in accordance with general chapter 2.6.37. Principles for the detection of extraneous viruses in immunological veterinary medicinal products using culture methods.
Methods using molecular techniques tend to be highly specific and therefore care must be taken to select suitable test parameters (e.g. PCR primers must be able to detect the various strains of an extraneous agent) and to consider the limitations of the technique. The results must be interpreted carefully.
ANNEX I: LIST OF EXTRANEOUS AGENTS TO BE CONSIDERED FOR THE RISK ASSESSMENT
| AVIAN (Poultry) – main list | |
| Viral agents | Bacterial agents |
| Atadenovirus (group III avian adenoviruses) | Avibacterium (Haemophilus) paragallinarum |
| Aviadenoviruses | Chlamydia spp. |
| Avian encephalomyelitis virus | Mycobacterium avium |
| Avian leucosis virus (excluding endogenous type) | Salmonella Pullorum |
| Avian metapneumovirus | |
| Avian nephritis virus (ANV) | |
| Avian orthoreoviruses | |
| Avian paramyxovirus type I | |
| Avian poxvirus | |
| Avian reticuloendotheliosis virus | |
| Avian rotavirus | |
| Infectious bursal disease virus type I and II | |
| Marek’s disease virus and meleagrid herpesvirus type 1 (HVT) | |
| Type A influenza virus | |
| AVIAN (additional list for Chicken) | |
| Viral agents | Bacterial agents |
| Avian infectious bronchitis virus | |
| Chicken anemia virus (CAV) | |
| Gallid herpesvirus type I | |
| AVIAN (additional list for Duck) | |
| Viral agents | Bacterial agents |
| Duck and goose parvoviruses | |
| Duck enteritis virus | |
| Duck hepatitis B virus (DHBV) | |
| Duck hepatitis virus type 1 | |
| AVIAN (additional list for Goose) | |
| Viral agents | Bacterial agents |
| Duck and goose parvoviruses | |
| Duck enteritis virus | |
| Goose haemorrhagic polyomavirus (GHPV) | |
| AVIAN (additional list for Turkey) | |
| Viral agents | Bacterial agents |
| Avian paramyxoviruses serotype 3 (APMV-3) | |
| Siadenovirus (group II avian adenovirus) | |
| Turkey coronavirus | |
| Turkey lymphoproliferative disease virus | |
| Turkey viral hepatitis virus | |
| AVIAN (additional list for Pigeon) | |
| Viral agents | Bacterial agents |
| Columbid herpesvirus 1 | |
| BOVINE | |
| Viral agents | Bacterial agents |
| Akabane virus | Brucella spp. |
| Alcelaphine herpesvirus | Chlamydia spp. |
| Bluetongue virus | Coxiella burnetii |
| Borna disease virus | Leptospira spp. |
| Bovine adenovirus | |
| Bovine coronavirus | |
| Bovine enterovirus | |
| Bovine ephemeral fever virus | |
| Bovine herpesvirus BHV-1 (IBR) | |
| Bovine leukaemia virus | |
| Bovine papillomavirus | |
| Bovine papular stomatitis virus | |
| Bovine parainfluenza virus 3 | |
| Bovine parvovirus | |
| Bovine polyomavirus | |
| Bovine respiratory syncytial virus | |
| Bovine rhinovirus | |
| Bovine viral diarrhoea virus | |
| Cache Valley virus | |
| Cowpox virus | |
| Endogenous retrovirus (replication competent) | |
| Epizootic haemorrhagic disease virus | |
| Foot-and-mouth disease virus | |
| Jena virus (Norwalk-like) | |
| Lumpy skin disease virus | |
| Ovine herpesvirus 2 (malignant catarrhal fever virus, European type) | |
| Pseudocowpox virus | |
| Rabies virus | |
| Reovirus | |
| Rift Valley fever virus | |
| Rinderpest virus | |
| Rotavirus | |
| Schmallenberg virus | |
| Swine herpesvirus 1 | |
| Tick-borne encephalitis virus | |
| Vesicular stomatitis virus | |
| Wesselsbron virus | |
| OVINE/CAPRINE | |
| Viral agents | Bacterial agents |
| Akabane virus | Brucella melitensis |
| Bluetongue virus | Brucella ovis |
| Border disease virus | Chlamydia spp. |
| Borna disease virus | Coxiella burnetii |
| Bovine viral diarrhoea virus | Leptospira spp. |
| Cache Valley virus | |
| Caprine herpesvirus | |
| Endogenous retrovirus (replication competent) | |
| Epizootic haemorrhagic disease virus | |
| Foot-and-mouth disease virus | |
| Maedi-Visna / caprine arthritis encephalitis virus | |
| Nairobi sheep disease virus | |
| Orf virus | |
| Ovine herpesvirus 2 (malignant catarrhal fever virus, European type) | |
| Ovine papilloma virus | |
| Ovine pulmonary adenocarcinoma virus (jaagsiekte) | |
| Ovine respiratory syncytial virus | |
| Ovine/caprine adenovirus | |
| Peste-des-petits-ruminants virus | |
| Rabies virus | |
| Rift Valley fever virus | |
| Sheeppox / goatpox virus | |
| Schmallenberg virus | |
| Swine herpesvirus 1 | |
| Tick-borne encephalitis virus | |
| Wesselsbron virus | |
| PORCINE | |
| Viral agents | Bacterial agents |
| African swine fever virus | Brucella suis |
| Bovine viral diarrhoea virus | Leptospira spp. |
| Classical swine fever virus | |
| Encephalomyocarditis virus | |
| Endogenous retrovirus (replication competent) | |
| Foot-and-mouth disease virus | |
| Hepatitis E virus | |
| Influenza virus | |
| Japanese encephalitis virus | |
| Nipah virus | |
| Porcine adenovirus | |
| PORCINE | |
| Viral agents | Bacterial agents |
| Porcine circovirus | |
| Porcine coronavirus (TGEV, PRCoV, PEDV) | |
| Porcine enterovirus | |
| Porcine parvovirus | |
| Porcine reproductive and respiratory syndrome virus | |
| Porcine rotavirus | |
| Rabies virus | |
| Swine herpesvirus | |
| Swinepox virus | |
| Vesicular stomatitis virus | |
| EQUINE | |
| Viral agents | Bacterial agents |
| African horse sickness virus | Burkholderia mallei |
| Borna disease virus | Burkholderia pseudomallei |
| Endogenous retrovirus (replication competent) | |
| Equine adenovirus | |
| Equine arteritis virus | |
| Equine encephalomyelitis alphavirus | |
| Equine encephalosis virus | |
| Equine herpesvirus (EHV-1, EHV-4) | |
| Equine infectious anaemia virus | |
| Equine influenza virus | |
| Equine rotavirus | |
| Hendra virus | |
| Japanese encephalitis virus | |
| Rabies virus | |
| Vesicular stomatitis virus | |
| West Nile virus | |
| CANINE | |
| Viral agents | Bacterial agents |
| Canid herpesvirus | Brucella canis |
| Canine adenovirus | Leptospira spp. |
| Canine coronavirus | |
| Canine distemper virus | |
| Canine oral papillomavirus | |
| Canine parainfluenza 2 virus | |
| Canine parvovirus | |
| Rabies virus | |
| Swine herpesvirus 1 |
| FELINE | |
| Viral agents | Bacterial agents |
| Cowpox virus | Chlamydia felis |
| Endogenous retrovirus (replication competent) | |
| Feline calicivirus | |
| Feline coronavirus | |
| Feline foamy virus (feline syncytia forming virus) | |
| Feline herpesvirus 1 | |
| Feline immunodeficiency virus | |
| Feline leukemia virus | |
| Feline panleucopenia virus | |
| Feline sarcoma virus | |
| Rabies virus | |
| Swine herpesvirus 1 | |
| RABBIT | |
| Viral agents | Bacterial agents |
| Arenavirus (lymphocytic choriomeningitis virus) | Francisella tularensis |
| Encephalomyocarditis virus | |
| Endogenous retrovirus (replication competent) | |
| Herpes simplex-like virus | |
| Leporid herpesvirus 2 | |
| Myxoma fibroma virus | |
| Rabbit enteric coronavirus | |
| Rabbit haemorrhagic disease virus | |
| Rabbit parvovirus | |
| Rabbitpox virus | |
| Rabies virus | |
| Rotavirus | |
| Swine herpesvirus 1 |
| RODENT (MOUSE) | |
| Viral agents | Bacterial agents |
| Ectromelia virus | Cilia-associated respiratory Bacillus |
| Endogenous retrovirus (replication competent) | Helicobacter spp. |
| Hantaan virus | |
| Kilham rat virus | |
| Lactic dehydrogenase-elevating virus | |
| Lymphocytic chorio-meningitis virus | |
| Minute virus of mice | |
| Mouse adenovirus | |
| Mouse cytomegalovirus | |
| Mouse encephalomyelitis virus | |
| Mouse hepatitis virus | |
| Mouse rotavirus | |
| Pneumonia virus of mice | |
| Polyomavirus | |
| Reovirus type 3 | |
| Thymic virus | |
| RODENT (HAMSTER) | |
| Viral agents | Bacterial agents |
| Endogenous retrovirus (replication competent) | Cilia-associated respiratory Bacillus |
| Lymphocytic chorio-meningitis virus | Helicobacter spp. |
| Pneumonia virus of mice | |
| Reovirus type 3 | |
| Sendai virus | |
| Simian virus type 5 | |
| RODENT (RAT) | |
| Viral agents | Bacterial agents |
| Endogenous retrovirus (replication competent) | Cilia-associated respiratory Bacillus |
| Hantaan virus | Helicobacter spp. |
| Kilham rat virus | |
| Mouse encephalomyelitis virus | |
| Pneumonia virus of mice | |
| Rat coronavirus/Sialoacryoadenitis virus | |
| Reovirus type 3 | |
| Sendai virus | |
| Toolan virus | |
| PRIMATES (VERO CELL) | |
| Viral agents | Bacterial agents |
| Bovine viral diarrhoea virus | |
| Endogenous retrovirus (replication competent) | |
| Herpesvirus | |
| Reovirus | |
| Simian virus 5 | |
| Simian virus 40 | |
| SALMONIDS | |
| Viral agents | Bacterial agents |
| Infectious haematopoietic necrosis virus (IHNV) | Aeromonas salmonicida |
| Infectious pancreatic necrosis virus (IPNV) | Fish-pathogenic Francisella spp. |
| Infectious salmon anaemia virus (ISAV) | Flavobacterium psychrophilum |
| Salmon alphaviruses | Piscirickettsia salmonis |
| Viral haemorrhagic septicaemia virus (VHSV) | Renibacterium salmoninarum |
| Vibrio anguillarum | |
| FINFISH | |
| Viral agents | Bacterial agents |
| Betanodavirus | Aeromonas salmonicida |
| Edwardsiella ictaluri | |
| Channel catfish virus | Fish-pathogenic Francisella spp. |
| Epizootic haematopoietic necrosis virus (EHNV) | Flavobacterium psychrophilum |
| Koi herpesvirus | Piscirickettsia salmonis |
| Oncorhynchous masou virus | Renibacterium salmoninarum |
| Perch rhabdovirus | Vibrio anguillarum |
| Red sea bream iridovirus | |
| Spring viraemia of carp virus (SVCV) | |
| Viral haemorrhagic septicaemia virus (VHSV) | |
ANNEX II: TESTING STRATEGY – EXAMPLE OF A DECISION TREE