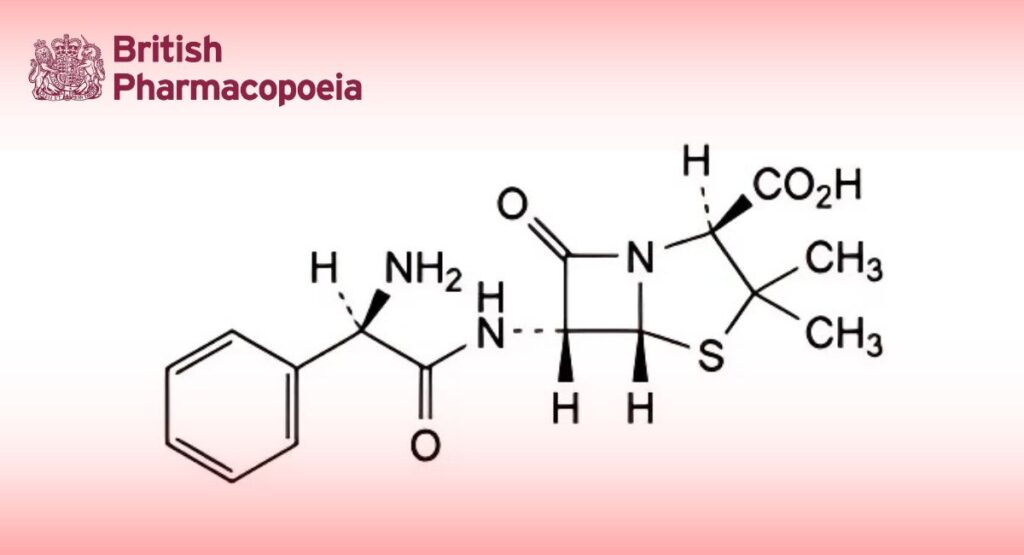
(Ph. Eur. monograph 0167)
C16H19N3O4S 349.4 69-53-4
Action and use
Penicillin antibacterial.
Preparations
Ampicillin Capsules
Ampicillin Oral Suspension
DEFINITION
(2S,5R,6R)-6-[[(2R)-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
Semi-synthetic product derived from a fermentation product.
Content
96.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, practically insoluble in acetone, in ethanol (96 per cent) and in fatty oils. It dissolves in dilute solutions of acids and of alkali hydroxides.
It shows polymorphism (5.9).
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs of potassium bromide R.
Comparison anhydrous ampicillin CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 25 mg of the substance to be examined in 10 mL of sodium hydrogen carbonate solution R.
Reference solution (a): Dissolve 25 mg of anhydrous ampicillin CRS in 10 mL of sodium hydrogen carbonate solution R.
Reference solution (b): Dissolve 25 mg of amoxicillin trihydrate CRS and 25 mg of anhydrous ampicillin CRS in 10 mL of sodium hydrogen carbonate solution R.
Plate: TLC silanised silica gel plate R.
Mobile phase: Mix 10 volumes of acetone R and 90 volumes of a 154 g/L solution of ammonium acetate R previously adjusted to pH 5.0 with glacial acetic acid R.
Application: 1 μL.
Development: Over a path of 15 cm.
Drying: In air.
Detection: Expose to iodine vapour until the spots appear and examine in daylight.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Place about 2 mg in a test-tube about 150 mm long and about 15 mm in diameter. Moisten with 0.05 mL of water R and add 2 mL of sulfuric acid-formaldehyde reagent R. Mix the contents of the tube by swirling; the solution is practically colourless. Place the test-tube in a water-bath for 1 min; a dark yellow colour develops.
D. Water (see Tests).
TESTS
Appearance of solution
The solutions are not more opalescent than reference suspension II (2.2.1).
Dissolve 1.0 g in 10 mL of 1 M hydrochloric acid. Separately dissolve 1.0 g in 10 mL of dilute ammonia R2. Examine immediately after dissolution.
pH (2.2.3)
3.5 to 5.5.
Dissolve 0.1 g in carbon dioxide-free water R and dilute to 40 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 280 to + 305 (anhydrous substance).
Dissolve 62.5 mg in water R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 27.0 mg of the substance to be examined in mobile phase A and dilute to 50.0 mL with mobile phase A.
Test solution (b): Prepare immediately before use. Dissolve 27.0 mg of the substance to be examined in mobile phase A and dilute to 10.0 mL with mobile phase A.
Reference solution (a): Dissolve 27.0 mg of anhydrous ampicillin CRS in mobile phase A and dilute to 50.0 mL with mobile phase A.
Reference solution (b): Dissolve 2.0 mg of cefradine CRS in mobile phase A and dilute to 50 mL with mobile phase A. To 5.0 mL of this solution add 5.0 mL of reference solution (a).
Reference solution (c): Dilute 1.0 mL of reference solution (a) to 20.0 mL with mobile phase A.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: mix 0.5 mL of dilute acetic acid R, 50 mL of 0.2 M potassium dihydrogen phosphate R and 50 mL of acetonitrile R, then dilute to 1000 mL with water R;
— mobile phase B: mix 0.5 mL of dilute acetic acid R, 50 mL of 0.2 M potassium dihydrogen phosphate R and 400 mL of acetonitrile R, then dilute to 1000 mL with water R;
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – tR | 85 | 15 |
| tR – (tR + 30) | 85 → 0 | 15 → 100 |
| (tR + 30) – (tR + 45) | 0 | 100 |
| (tR + 45) – (tR + 60) | 85 | 15 |
tR = retention time of ampicillin determined with reference solution (c)
If the mobile phase composition has been adjusted to achieve the required resolution, the adjusted composition will apply at time zero in the gradient and in the assay.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 50 μL of reference solutions (b) and (c) with isocratic elution at the initial mobile phase composition and 50 μL of test solution (b) according to the elution gradient described under Mobile phase; inject mobile phase A as a blank according to the elution gradient described under Mobile phase.
System suitability: Reference solution (b):
— resolution: minimum 3.0 between the peaks due to ampicillin and cefradin; if necessary, adjust the ratio A:B of the mobile phase.
Limit:
— any impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (c) (1.0 per cent).
N,N-Dimethylaniline (2.4.26, Method B)
Maximum 20 ppm.
Water (2.5.12)
Maximum 2.0 per cent, determined on 0.300 g.
Sulfated ash (2.4.14)
Maximum 0.5 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase Initial composition of the mixture of mobile phases A and B, adjusted where applicable.
Injection: Test solution (a) and reference solution (a).
System suitability: Reference solution (a):
— repeatability: maximum relative standard deviation of 1.0 per cent after 6 injections.
Calculate the percentage content of C16H19N3O4S from the declared content of anhydrous ampicillin CRS.
STORAGE
In an airtight container, at a temperature not exceeding 30 °C.
IMPURITIES
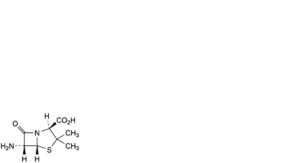
A. (2S,5R,6R)-6-amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (6-aminopenicillanic acid),
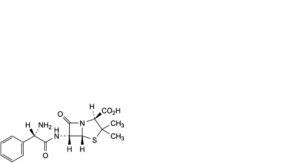
B. (2S,5R,6R)-6-[[(2S)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (L-ampicillin),
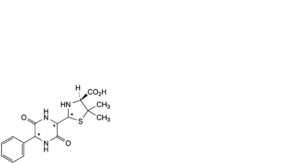
C. (4S)-2-(3,6-dioxo-5-phenylpiperazin-2-yl)-5,5-dimethylthiazolidine-4-carboxylic acid (diketopiperazines of ampicillin),
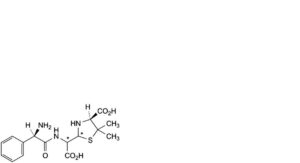
D. (4S)-2-[[[(2R)-2-amino-2-phenylacetyl]amino]carboxymethyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid (penicilloic acids of ampicillin),
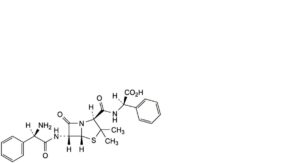
E. (2R)-2-[[[(2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl]amino]-2-phenylacetic acid (ampicillinyl-D-phenylglycine),
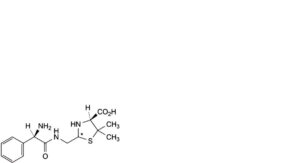
F. (2RS,4S)-2-[[[(2R)-2-amino-2-phenylacetyl]amino]methyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid (penilloic acids of ampicillin),
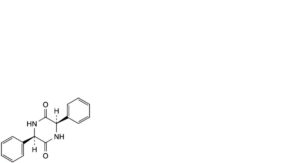
G. (3R,6R)-3,6-diphenylpiperazine-2,5-dione,

H. 3-phenylpyrazin-2-ol,
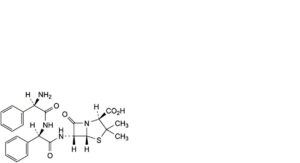
I. (2S,5R,6R)-6-[[(2R)-2-[[(2R)-2-amino-2-phenylacetyl]amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (D-phenylglycylampicillin),
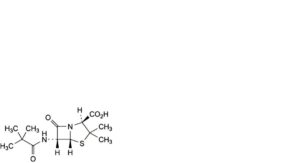
J. (2S,5R,6R)-6-[(2,2-dimethylpropanoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,

K. (2R)-2-[(2,2-dimethylpropanoyl)amino]-2-phenylacetic acid,

L. (2R)-2-amino-2-phenylacetic acid (D-phenylglycine),
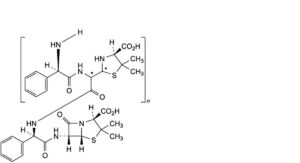
M. co-oligomers of ampicillin and of penicilloic acids of ampicillin.