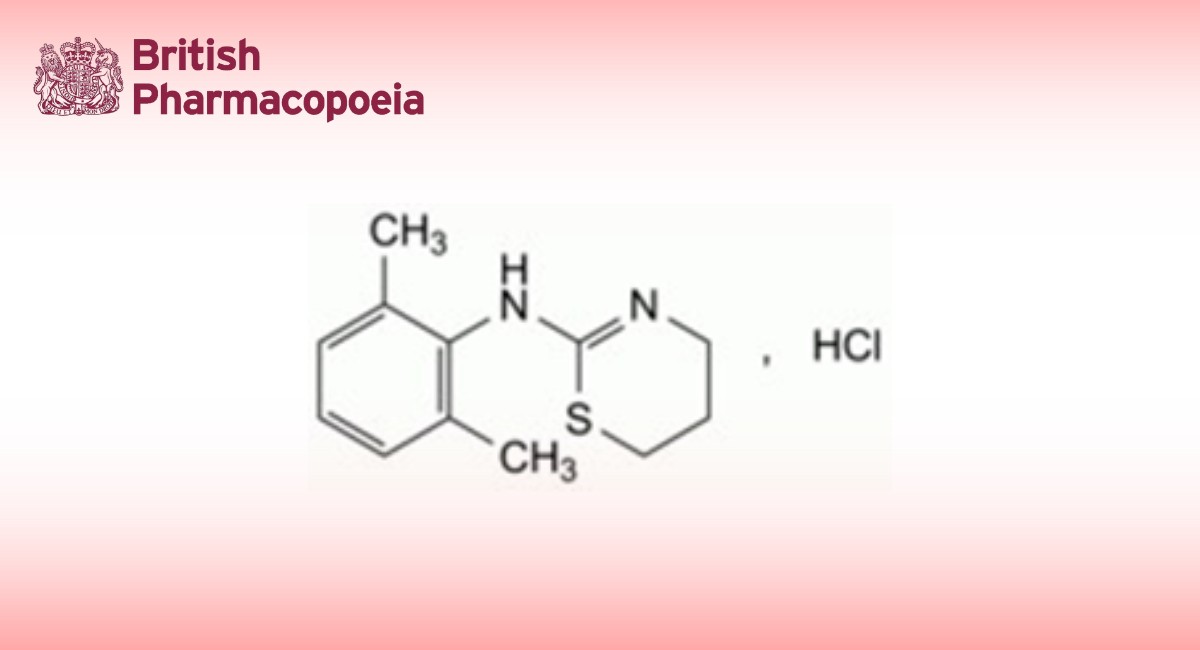
Action and use
Benzimidazole antihelminthic.
DEFINITION
Albendazole Oral Suspension with Minerals is a suspension of Albendazole in a suitable vehicle containing cobalt and selenium.
The oral suspension complies with the requirements stated under Oral Liquids and with the following requirements.
Content of albendazole, C12H15N3O2S
95.0 to 105.0% of the stated amount.
Content of Co
90.0 to 110.0% of the stated amount.
Content of Se
90.0 to 115.0% of the stated amount.
IDENTIFICATION
A. To a volume of oral suspension containing of 25 mg of Albendazole add 50 mL of 0.1M sodium hydroxide and shake with the aid of ultrasound for 10 minutes. Dilute to 100 mL with the same solvent, filter through a 0.45-μm filter and dilute 1 volume of this solution to 10 volumes with the same solvent. The light absorption, Appendix II B, in the range 240 to 340 nm of the resulting solution exhibits a maximum at 308 nm, a minimum at 281 nm and a shoulder at 269 nm.
B. To a volume of oral suspension containing of 25 mg of Albendazole add 50 mL of 0.1M hydrochloric acid and shake with the aid of ultrasound for 10 minutes. Dilute to 100 mL with the same solvent, filter through a 0.45-μm filter and dilute 1 volume of this solution to 10 volumes with the same solvent. The light absorption, Appendix II B, in the range 240 to 340 nm of the resulting solution exhibits a maximum at 292 nm, a minimum at 273 nm and a shoulder at 261 nm.
C. In the Assay for albendazole, the chromatogram obtained with solution (1) shows a peak with the same retention time as the principal peak in the chromatogram obtained with solution (2).
D. In the Assay for cobalt, the oral suspension absorbs radiation at 240.7 nm.
E. In the Assay for selenium, the oral suspension absorbs radiation at 196.0 nm.
TESTS
Acidity
pH, 4.5 to 6.0, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solvent A: 30% of mobile phase A and 70% of mobile phase B.
(1) Dilute a quantity of the oral suspension with methanolic sulfuric acid (1%) to give a solution containing 1.0% w/v of Albendazole and dilute 1 volume to 2 volumes with solvent A.
(2) Dilute 1 volume of solution (1) to 100 volumes with solvent A.
(3) Dissolve 25.0 mg of albendazole BPCRS and 25.0 mg of oxibendazole BPCRS in 5 mL of 1% v/v solution of methanolic sulfuric acid and dilute to 50 mL with solvent A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 μm) (Waters Symmetry is suitable).
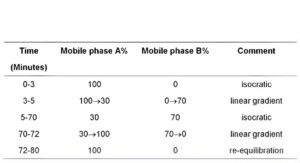
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 0.7 mL per minute.
(d) Use ambient column temperature.
(e) Use a detection wavelength of 292 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A: 0.015M ammonium dihydrogen orthophosphate.
Mobile phase B: methanol.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks is at least 7.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%);
the sum of the areas of any secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (2%).
Disregard any peak with an area less than 0.05 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
ASSAY
For albendazole
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Add 70 mL of 1% v/v solution of methanolic sulfuric acid to a quantity of the oral suspension containing 0.10 g of Albendazole, stir for 15 minutes, mix with the aid of ultrasound for 10 minutes and add sufficient 1% v/v solution of methanolic sulfuric acid to produce 100 mL. Allow to stand and dilute 5 volumes of the clear supernatant to 25 volumes with 1% v/v solution of methanolic sulfuric acid.
(2) 0.020% w/v of albendazole BPCRS in 1% v/v solution of methanolic sulfuric acid.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
DETERMINATION OF CONTENT
Determine the weight per mL of the oral suspension, Appendix V G, and calculate the content of C12H15N3O2S, weight in volume, using the declared content of C12H15N3O2S in albendazole BPCRS.
For cobalt
Add 5 mL of hydrochloric acid to a quantity of the oral suspension containing 2.5 mg of cobalt, heat in a water bath for 10 minutes, cool and add sufficient water to produce 100 mL. Mix thoroughly and filter. Carry out the method for atomic absorption spectrophotometry, Appendix II
D, measuring at 240.7 nm and using cobalt standard solution (100 ppm Co), diluted if necessary with water, to prepare the standard solution. Determine the weight per mL of the oral suspension, Appendix V G, and calculate the content of Co, weight in volume.
For selenium
Add 5 mL of nitric acid to a quantity of the oral suspension containing 2.5 mg of selenium, heat in a water bath for 15 minutes, cool and add sufficient water to produce 50 mL. Mix thoroughly and filter. Carry out the method for atomic absorption spectrophotometry, Appendix II D, measuring at 196.0 nm and using selenium standard solution (100 ppm Se), diluted if necessary with water, to prepare the standard solution. Determine the weight per mL of the oral suspension, Appendix V G, and calculate the content of Se, weight in volume.